Poloxamer 188 not effective for acute management of vaso-occlusive episodes in patients with sickle cell disease
1. Poloxamer 188 did not decrease parenteral opioids reliance for pain control during acute vaso-occlusive episodes in adults and children with sickle cell disease.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Sickle cell disease is a group of inherited hemoglobinopathies affecting patients worldwide and is the most common genetic disease in the United States. Painful vaso-occlusive episodes are a hallmark feature of this multisystem disorder. Although there are FDA approved treatment for prevention of vaso-occlusive episodes, there is no effective agents available once the vaso-occlusive episode has started. It has been theorized that poloxamer 188 (vepoloxamer, MST-188, RheothRx), a nonionic block polymer surfactant, could help treat acute vaso-occlusive episode based on its potential antithrombotic and anti-inflammatory properties. Participants were recruited from 46 sites in the US and 20 sites in 11 other countries between May 2013 and February 2016 and randomly assigned to the treatment group (Poloxamer 188) and the placebo group. There was no significant difference between the two groups in terms of time until last administration of parenteral opioids. One strength of this study was its double-blind, placebo-controlled randomized design at multiple centers worldwide. It is difficult to completely blind the administration of the poloxamer 188 infusion, potentially confounding the placebo-controlled nature of the study. The outcome measure is also relatively subjective; the decision to discontinue opioid medication can be influenced by many factors such as patient emotion and personal prevalence.
Click to read the study in JAMA
Click to read an accompanying editorial in JAMA
Relevant Reading: Pharmacological interventions for painful sickle cell vaso‐occlusive crises in adults
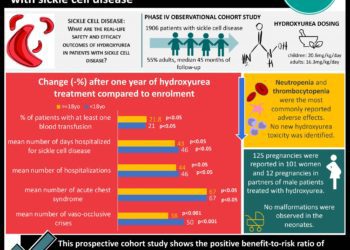
In-Depth [randomized controlled trial]: In this phase 3, double-blind, placebo-controlled randomized international clinical trial, adults and children with sickle cell disease were treated with Poloxamer 188 infusion or placebo. The mean time from randomization to last dose of parenteral opioids was 81.8 hours in the treatment group and 77.8 hours in the placebo group. There is no significant differences in the two groups with a 95% confidence interval (P = .09). Notably, more participants in the treatment group developed significant adverse events; 32 participants (16.5%) had developed acute chest syndrome when received Poloxamer 188 infusion while the incidence was only 22 participants in the placebo group (1.2%; difference 5.2 [95% CI –1.7-12.0]). The study did not support Poloxamer 188 as an effective agent for acute painful vaso-occlusive episodes.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.