Prophylactic letermovir significantly reduces cytomegalovirus infection after transplantation
1. Compared to placebo, letermovir prophylaxis significantly decreased the incidence of cytomegalovirus (CMV) infection through 24 weeks following hematopoietic stem cell transplantation (HSCT).
2. Letermovir was associated with a significantly lower all-cause mortality compared to placebo and did not demonstrate any serious toxicities.
Evidence Rating Level: 1 (Excellent)
Study Rundown: While many centers use serial CMV testing to assess for viremia as a preemptive strategy to detect CMV infection post-HSCT, prophylactic antiviral therapy is used in certain high-risk patients. This decision is made on a case-by-case basis by balancing the efficacy of the antiviral and the potential toxicities. This reports the results of a phase 3 trial of the anti-CMV agent letermovir, which follows a phase 2 dose-escalation trial demonstrating the efficacy of letermovir in preventing CMV viremia after HSCT. The primary endpoint, clinically significant CMV infection through week 24 following transplantation, was significant reduced with letermovir compared to placebo. Letermovir also demonstrated a significant reduction in all-cause mortality without any significant toxicities compared to placebo. Letermovir may represent an effective agent for prevention of CMV infection after HSCT, while avoiding the myelotoxic and nephrotoxic effects of other common antiviral agents.
Limitations of this trial include using a lower threshold of CMV viremia than what is commonly used in clinical practice. Furthermore, the study’s definition of high-risk patients was subjective and the study’s duration may not have been sufficient to uncover drug interactions and potential long-term toxicities associated with letermovir. Thus, the decision to give prophylactic therapy will likely continue to be made on a case-by-case basis.
Click to read the study in NEJM
Relevant Reading: Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies
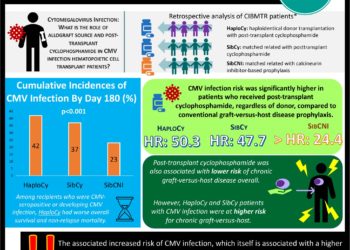
In-Depth [randomized controlled trial]: This was a multicenter, double-blinded, phase 3 clinical trial that randomized 495 patients in a 2:1 ratio to letermovir (n=325) (480 mg or 240 mg if already receiving concomitant cyclosporine) or placebo (n=170). Randomization was stratified according to CMV disease risk, with high-risk meeting one or more of the following criteria: at least one human leukocyte antigen (HLA) mismatch in related (three specified HLA loci) or unrelated (four specified loci) donor, haploidentical donor, use of umbilical cord blood as stem-cell source, use of ex-vivo T-cell depleted grafts, or use of at least 1mg prednisone due to grade 2 or higher graft-versus-host disease. Preemptive antiviral treatment was initiated for CMV viremia of >150 copies/mL in high-risk and >300 copies/mL in low-risk patients. The primary endpoint was the proportion of patients with clinically significant CMV infection through week 24 after transplantation. Secondary endpoints included the proportion of patients with clinically significant CMV infection through week 14, all-cause mortality, and safety data.
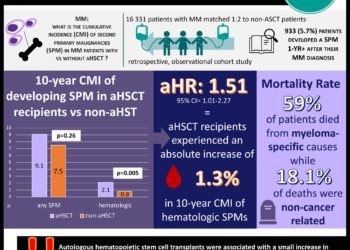
In the 495 patients included in the primary efficacy endpoint, recipients of letermovir (122 of 325, 37.5%) demonstrated significantly lower rates of clinically significant CMV infection than those in the placebo (103 of 170, 60.6%) group (difference -23.5%; 95% confidence interval [CI], -32.5 to -14.6%; P<0.001). CMV disease was uncommon in both groups (1.5% in letermovir and 1.8% in placebo). Patients in the letermovir group also demonstrated significantly lower rates of CMV infection at 14 weeks post-transplantation (19.1% vs. 50.0%; -31.3% difference; 95% CI, -39.9 to -22.6%; P<0.001). All-cause mortality at week 24 post-transplantation was lower with letermovir versus placebo (10.2% [95% CI, 6.8 to 13.6%] vs. 15.9% [95% CI 10.2 to 21.6%]; P=0.03). The frequency and severity of adverse events were similar in the two groups overall. There was a non-significant increased incidence of vomiting, edema, and atrial fibrillation and flutter in patients who received letermovir. There was no difference in adverse events related to myelosuppression or nephrotoxicity.
Image: PD
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc