Psilocybin does not significantly improve depressive scores compared to escitalopram
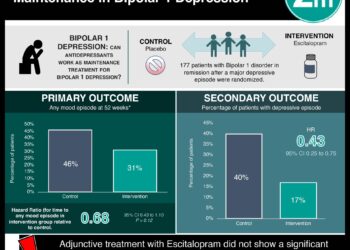
1. Psilocybin treatment was shown not to report significantly improved self-reported depression scores compared to escitalopram treatment in patients with major depressive disorder.
2. Secondary outcomes of other mental health scales may have improved with psilocybin treatment but could not be directly compared to escitalopram treatment.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Psilocybin is a natural serotonin 5-hydroxytryptamine type 2A receptor antagonist found in psychoactive mushrooms. This study tested a course of psilocybin compared to standard therapy of escitalopram, a selective serotonin receptor inhibitor (SSRI), for the treatment of the major depressive disorder. Compared to the escitalopram group, the study found no significant improvement in self-reported depression scores as measured by the 16-item Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR-16) after six weeks. Other depression and mental assessment questionnaires that were used appeared to favor the psilocybin group, but a direct comparison could not be made as they were not adjusted for multiple comparisons. No serious adverse events were reported in either group. The strength of this study was the comprehensive array of different measures of mental well-being ranging from anxiety to work/social adjustment to the ability to feel emotion. One major limitation to this study was the short duration of escitalopram treatment, as efficacy has been documented to be improved after an extended duration. Nonetheless, the study’s results are significant as psilocybin and escitalopram do not vary in treatment effect based on the QIDS-SR-16 score.
Click to read the study in NEJM
Relevant Reading: The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis
In-Depth [randomized controlled trial]: This randomized control trial enrolled 59 patients to compare the depression scores in patients with psilocybin and escitalopram treatment. Patients included in the study were men and women diagnosed with moderate-to-severe major depressive disorder between 18 and 80 years of age. Patients with immediate family or personal history with psychosis, a positive pregnancy test, or a history of serious suicide attempts were excluded from the study. Patients were randomized in a 1:1 ratio to receive either psilocybin or placebo treatment, respectively. The primary outcome of the study was changed from baseline in QIDS-SR-16 scores. A higher QIDS-SR-16 score indicated greater depression. The study found no significance in QIDS-SR-16 score in the psilocybin group (mean ± SE, 8.0 ± 1.0) and the control group (mean ± SE, 6.0 ± 1.0). The between-group difference was 2.0 points (95% confidence interval [CI], -5.0 to 0.9, P = 0.17). In addition, patients receiving psilocybin reported an improvement in a majority of secondary outcome measures including feelings of well-being, as measured by the Warwick-Edinburgh Mental Wellbeing scale (WEMWBS), by 8.1 points and decreased anxiety, as measured by the Spielberger’s Trait Anxiety Inventory (STAI), by 9.0 points. However, direct comparisons on this improvement could not be made as the confidence intervals were not adjusted for multiple comparisons. Overall, psilocybin and escitalopram treatments did not show any significant changes in QIDS-SR-16 score for long-standing, moderate-to-severe major depressive disorder.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.