Quick Take: Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent)
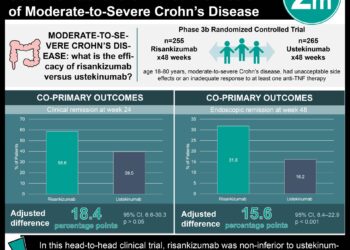
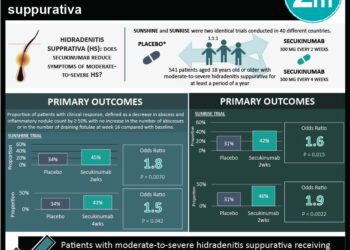
In this randomized controlled trial, 605 patients with moderate-to-severe chronic plaque psoriasis were assigned to receive 150 mg risankizumab subcutaneously at weeks 0 and 4, or 80 mg of adalimumab subcutaneously, and then 40 mg at weeks 1, 3, 5 and every other week thereafter during a 16-week treatment period to evaluate the efficacy and safety of risankizumab compared with adalimumab in this patient population. After 16 weeks, adalimumab intermediate responders were re-randomized to receive either continue 40 mg adalimumab or to switch to 150 mg risankizumab. Researchers found that at 16 weeks of follow-up, a 90% reduction in Psoriasis Area and Severity Index (PASI-90) was achieved in 72% of patients in the risankizumab group and in 47% of patients given adalimumab (difference 24.9%, 95% CI 17.5% to 32.4%, p<0.0001). In addition sPGA (static Physician Global Assessment) scores of 0 or 1 were achieved in 84% of patients given risankizumab, compared to 60% of patients in the adalimumab group (difference 23.3%, 95% CI 16.6% to 30.1%, p<0.0001). In the second part of the study, PASI-90 was observed in 66% of patients that switched to risankizumab versus 21% of patients that continued with adalimumab at 44 weeks of follow-up (difference 45.0%, 95% CI 28.9 to 61.1%, p<0.0001). The incidence of adverse events was comparable between groups. This study therefore shows that risankizumab demonstrates significantly greater efficacy compared to adalimumab in patients with moderate-to-severe plaque psoriasis, with comparable safety profile.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.