Rate of change of Antimüllerian hormone (AMH) is an independent predictor of time to menopause in late reproductive age women
Key study points:
1. In women aged 35-48 years old, rate of AMH change was independently associated with risk of menopause.
2. Including rate of AMH change with baseline AMH level and age increased the precision of estimates of time to menopause.
Primer: Menopause is defined as the cessation of menstruation for 12 months, which represents complete or near complete ovarian follicular depletion. Perimenopause, or the menopausal transition, represents the shift from regular ovulatory cycles to permanent infertility. In the United States, the median age of menopause is 51.4 years and of perimenopause is about 46 (1).
Accurately diagnosing the menopausal transition is clinically necessary when women present for infertility evaluation (3) or when they present with symptoms of peri-menopause (e.g. amenorrhea, vasomotor symptoms) that overlap with other conditions such as hyperthyroidism, pregnancy, carcinoid and hyperprolactinemia. In either setting, the Staging of Reproductive Aging Workshop (STRAW) staging criteria can be used as a tool to assess ovarian reserve in infertility patients or to approximate time to menopause, though the criteria (antral follicle count, follicle-stimulating hormone (FSH), anti-müllerian hormone (AMH) and inhibin B levels) have not been validated for use in the evaluation of menopausal status (2). In a woman undergoing the menopausal transition, FSH is high due to disinhibition of the anterior pituitary while AMH, inhibin B and AFC are low because the ovaries produce less hormones (low AMH, inhibin B and estrogen) and less follicles are recruited in a hypoestrogenic setting.
In the ART population, fertility specialists rely on FSH and AMH as serum markers of ovarian reserve. Historically, measuring FSH and estrogen levels on Day 3 and 10 after a clomid challenge test has been standard practice for approximating ovarian reserve (3). However, in 2001 research showed that AMH may be a more reliable marker (4). AMH is further favorable because it is not cycle-dependent such that it can be measured at any time in a woman’s cycle. Further, AMH measurement does not require an antecedent clomid challenge, as does measurement of FSH (5).
The present work is the first to assess the rate of AMH change as a predictor of ovarian follicular atresia.
Background reading:
- Up-to-Date: Diagnosis of Menopause
- Executive Summary: Staging Reproductive Aging
- Up-to-Date: Female Infertility
- AMH: a putative marker of ovarian aging
- AMH better predictive marker than FSH
This [prospective cohort] study evaluated 293 premenopausal women in the Penn Ovarian Aging Study who had both a detectable baseline AMH level (at least 0.20ng/mL) and at least one additional AMH measure 1 or more years later. The cohort was randomly identified by telephone and stratified to obtain equal numbers of black and white women.
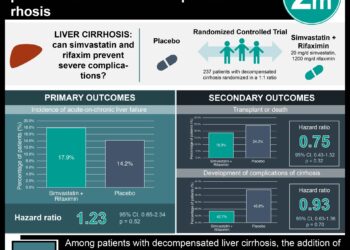
Overall, results showed that including rate of AMH change along with baseline AMH level and age increased the precision of estimates of time to menopause. The rate of AMH change was associated with time to menopause in late reproductive age women (35-48 years old) such that women with similar baseline AMH levels experienced time to menopause that differed by about 2 years between those with a slow versus fast rate of AMH change. Interestingly, women in the 35-39 age group with faster decrease in AMH experienced a dramatically increased risk of menopause (Hazard ratio: 6.97, 95% CI 3.81-12.72) while women in the 40-44 and 45-48 age groups experienced a less dramatic but still significant associations.
In sum: The results of the present work suggest that the rate of AMH change may be a more direct surrogate than age or single AMH measurement in predicting time to menopause.
Click to read this article in the current issue of Fertility and Sterility
By [LH]
© 2012 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. DISCLAIMER: Posts are not medical advice and are not intended as such. Please see a healthcare professional if you seek medical advice.