Rivaroxaban potentially inferior to vitamin K antagonists for treatment of thrombotic antiphospholipid antibody syndrome
1. In this randomized study comparing the efficacy of rivaroxaban with warfarin, dose-adjusted rivaroxaban treatment resulted in near doubling of risk for recurrent thrombosis.
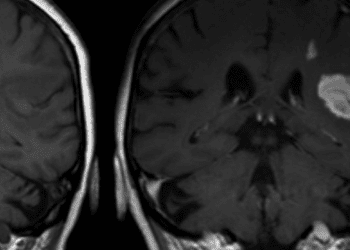
2. The two treatment groups experienced similar bleeding outcomes, but patients in the rivaroxaban group had a higher stroke incidence rate.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Antiphospholipid Antibody Syndrome (APS) is a rare thrombophilic disorder treated primarily through long-term anticoagulation with vitamin K antagonists (VKA). However, this method has several notable disadvantages, including the need for frequent monitoring, the likelihood of food and drug interactions, and the increased risk of bleeding complications. With the advent of novel anticoagulation therapies, it is necessary to reevaluate whether this strategy ought to remain a mainstay amongst numerous alternatives. In this open-label noninferiority study, rivaroxaban failed to demonstrate noninferiority to warfarin for thrombotic APS, showing a non-statistically significant near doubling of risk for recurrent thrombosis. These findings were consistent with a previous rivaroxaban noninferiority study that showed not only a higher rate of thrombotic events but also a greater frequency of occurrence in arterial circulation. Additionally, stroke incidence was substantially higher in the rivaroxaban group. Limitations of this study included failure to assess anticoagulation intensity, reliance on exploratory post hoc analyses, and a small sample size with insufficient power to detect differences between patient subgroups.
Click to read the study in Annals of Internal Medicine
Click to read an accompanying editorial in Annals of Internal Medicine
Relevant Reading: The Management of Thrombosis in the Antiphospholipid-Antibody Syndrome
In-Depth [randomized controlled trial]: This randomized open-label phase 3 trial was conducted at 6 university hospitals in Spain and recruited 190 patients according to the international consensus criteria for APS. Inclusion criteria were objectively confirmed arterial or venous thrombosis as well as repeated positive results upon testing for the presence of one or more antiphospholipid antibodies. Exclusion criteria were clinically significant bleeding diathesis, recent stroke or intracranial/gastrointestinal hemorrhage, pregnancy, renal impairment, Child-Pugh class B or C cirrhosis, or documented nonadherence to existing warfarin regimen. For patients assigned to receive rivaroxaban, therapeutic low-molecular-weight heparin was used as a transitional intermediate before initiating treatment.
The primary efficacy endpoint was the proportion of patients with a thrombotic event, and secondary endpoints included time to thrombosis, type of thrombotic event, and changes in levels of selected biomarkers. The primary safety endpoint was the proportion of patients with a major hemorrhagic episode. The noninferiority limit of 1.40 was established based on meta-analysis data comparing warfarin versus placebo. With 95 patients enrolled in each group, statistical power was limited to 80%. In per protocol analysis, recurrent thrombosis occurred in 11 patients (11.6%) in the rivaroxaban group and 6 in the VKA group (RR, 1.83 [CI, 0.71 to 4.76]; P for noninferiority = 0.29, P for VKA superiority = 0.20). Major bleeding occurred in 6 patients (6.3%) in the rivaroxaban group and 7 (7.4%) in the VKA group (corrected RR, 0.86 [CI, 0.30 to 2.46]).
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.