Sacituzumab govitecan increased survival in metastatic triple-negative breast cancer
1. Sacituzumab govitecan was shown to significantly increase overall survival in previously treated metastatic triple-negative breast cancer patients compared to single-agent chemotherapy treatment.
2. Sacituzumab govitecan was associated with more frequent events of myelosuppression and diarrhea compared to chemotherapy treatment.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Breast cancer patients with triple-negative status have significantly worse outcomes in the metastatic settings compared to their receptor-positive counterparts. In triple-negative patients that progress on chemotherapy, single-agents are the next step; however, the response is poor. Sacituzumab govitecan is an antibody-drug conjugate that targets antitrophoblast cell-surface antigen 2, a receptor that is highly expressed in breast cancer, coupled with SN-38, a metabolite of the chemotherapy, irinotecan, a topoisomerase I inhibitor. In this trial of metastatic triple-negative breast cancer patients who had progressed on two or more previous standard chemotherapy regimens, patients were randomized to sacituzumab govitecan or a pre-determined standard of care single-agent chemotherapy. Sacituzumab govitecan demonstrated superior progress-free survival and overall survival compared to single-agent chemotherapy. Adverse events were higher in the sacituzumab govitecan group and included neutropenia, diarrhea, anemia, and febrile neutropenia. The study limitation was the heterogeneity of the chemotherapy drugs, which may have contributed to variable toxicity profiles in the comparator group. In general, this clinical trial demonstrated sacituzumab govitecan was superior for prolonging overall survival compared to single-agent chemotherapy.
Click here to read the study in the NEJM
Relevant Reading: Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC)
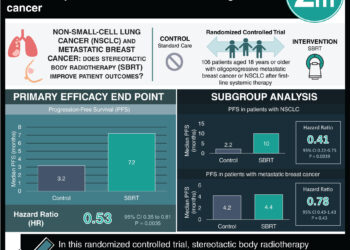
In-Depth [randomized controlled trial]: This was a multicenter, randomized, open-label trial of 468 patients at 88 sites in seven countries. Patients with metastatic triple-negative breast cancer who progressed on two or more standard chemotherapy lines of therapy including a taxane in any indication were included in the study. Patients with brain metastases were excluded from the primary analysis. Patients were randomized in a 1:1 ratio to receive the sacituzumab govitecan or single-agent chemotherapy such as eribulin, vinorelbine, capecitabine, or gemcitabine, respectively. The primary endpoint was progression-free survival. Sacituzumab govitecan (5.6 months) had a significantly higher progression-free survival compared to the control group (1.7 months) (hazard ratio for progression or death [HR]; 0.41, 95% confidence interval [CI], 0.32 to 0.52; P < 0.001). Furthermore, sacituzumab govitecan (12.1 months) showed significantly longer overall survival compared to the control group (6.7 months) (HR, 0.48; 95% CI, 0.38 to 0.59; P < 0.001). Grade 3 or higher adverse events were higher in sacituzumab govitecan group, which included more neutropenia (sacituzumab govitecan, 51%; chemotherapy, 33%), diarrhea (sacituzumab govitecan, 10%; chemotherapy, <1%), and anemia (sacituzumab govitecan, 8%; chemotherapy, 5%). Treatment discontinuations were similar between both groups. Overall, this large clinical trial demonstrated the efficacy of using an antibody-drug conjugate in cancer treatment to prolong overall survival in metastatic triple-negative breast cancer.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.