SALT-ED: Normal Saline versus Balanced Crystalloids in Noncriticaly Ill Adults [Classics Series]
This study summary is an excerpt from the book 2 Minute Medicine’s The Classics in Medicine: Summaries of the Landmark Trials
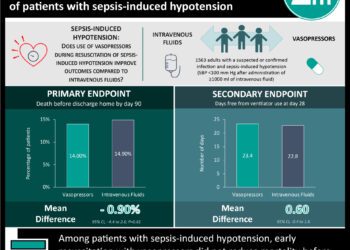
1. This trial found no difference in a composite measurement of in-hospital death and length of stay between normal saline and balanced crystalloids in noncritically ill adults.
2. Major adverse renal events were lower in incidence at 30 days in the balanced crystalloids group.
Original Date of Publication: March 2018
Study Rundown: The SALT-ED study was a landmark trial demonstrating no difference in hospital-free days at day 28 (a composite measurement of in-hospital death and length of stay) in noncritically ill adults when receiving normal saline versus balanced crystalloids (Ringer’s Lactate or Plasma-Lyte A). Eligible adults had received at least 500mL of fluid in the emergency department before being admitted to hospital outside of the intensive care unit (ICU). The median hospital-free days was 25 and did not differ significantly between the two groups. Of note was the significant difference in major renal adverse events at 30 days between balanced crystalloid (4.7%) versus normal saline (5.6%), with a corresponding number needed to treat of 111. As balanced crystalloids are largely similar to normal saline in availability and cost, the authors present this paper as support for the use of the former in general fluid resuscitation. A major strength of this study was high adherence to the assigned fluid group; likewise, from a pragmatic standpoint, the unblinded trial design facilitated the use of the assigned fluid group immediately in acute situations. Limitations of the study included its single center design, limiting its generalizability. Likewise, its pragmatic design prevented the collection of detailed patient data to further stratify participants by characteristics. Finally, a major shortcoming was that fluid administration was recorded only in the emergency department before admission into hospital, despite outcome measurements lasting 4 weeks.
Click to read the study in NEJM
In-Depth [randomized control trial]:The SALT-ED trial was conducted between January 2016 and April 2017 in a high-volume, tertiary-care, academic, hospital-based emergency department in the United States. Eligible patients were adults in the emergency department having received at least 500mL of intravenous fluid and subsequently admitted to hospital outside of the ICU. Given that this was a crossover trial, the fluid used was swapped each month for all patients over the study period. Overall, 13 347 patients were eligible for inclusion over this 16 month period. No significant difference was found in hospital-free days between the two groups, with a median of 25 days (p = 0.41). Importantly, patients in the balanced crystalloid group did have a lower incidence of major renal adverse events at 30 days (4.7% vs. 5.6%; p = 0.01). These events included new renal-replacement therapy, significantly elevated serum creatinine, or stage 2 or higher acute kidney injury.
Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. New England Journal of Medicine. 2018 Mar 1;378(9):819–28.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.