Selective estrogen receptor modulators reduce breast cancer risk even after treatment
Image: PD
1. Selective estrogen receptor modulators (SERMs) reduce the risk of ER-positive invasive breast cancer in high-risk women for at least 5 years after completion of standard 5-year treatment course (at 10 year follow-up).
2. SERM benefit in reducing cancer incidence was greater in the 5 years during treatment as compared to the 5 years after treatment, though reductions were significant in both time periods.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Researchers found that SERMs significantly reduced the risk of all breast cancer, mainly by reducing the incidence of ER-positive invasive cancers, in high-risk and average-risk women for at least 5 years after the completion of the standard 5-year SERM treatment course. This study is the first to analyze all SERM prevention trials and is one of the only investigations to include longer-term follow-up (up to 10 years).
A major strength is the use of individual participant data, which allowed researchers to perform statistical analyses that otherwise could not be done in a meta-analysis. Results are limited by the lack of long-term follow-up data, particularly for arzoxifene and lasofoxifene. Further studies should be aimed at evaluating longitudinal benefits of the less well-studied SERMs, as well as even longer-term follow-up for all SERMs to assess the full duration of their protective effects.
Click to read the study in The Lancet
Click to read an accompanying editorial in The Lancet
Relevant Reading: Uptodate: Selective estrogen receptor modulators and aromatase inhibitors for breast cancer prevention
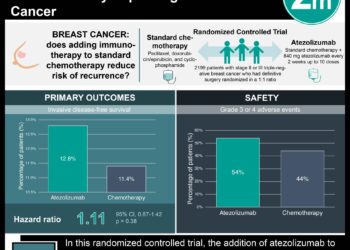
In-Depth [meta-analysis]: Researchers identified nine randomized controlled trials that looked at breast cancer incidence and medication side effects in women taking various selective estrogen receptor modulators (SERMs; tamoxifen, raloxifene, arzoxifene, and lasofoxifene). Individual participant data was obtained from each trial investigator and analysis was done by intention to treat. The primary outcome was incidence of all breast cancer (including ductal carcinoma in situ) at 10 years. Secondary outcomes included incidence of other cancers, venous thromboembolic events (VTE), cardiovascular events, fractures, cataracts, and all-cause mortality.
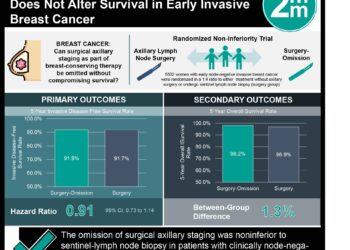
In a meta-analysis combining data from nine trials, 83,399 participants with a total of 306,617 women-years of follow-up were included. At 10 year follow-up, women on a SERM experienced a 38% reduction (Hazard ratio [HR] 0.62, 95% CI 0.56-0.69) in breast cancer incidence at 10 years compared to women on a control, resulting in 42 women needed to treat to prevent one case of breast cancer. SERM benefit in reducing cancer incidence was greater in the 5 years during treatment as compared to the 5 years after treatment, though reductions were significant in both time periods. The incidence of VTEs was significantly higher with SERMs (OR 1.73, 95% CI 1.47-2.05; p<0.0001), while the incidence of vertebral fractures was significantly lower (OR 0.66, 95% CI 0.59-0.73). No significant differences were seen in all-cause mortality.
By Maren Shapiro and Leah Hawkins
More from this author: USPSTF recommends chemoprevention for women at high risk for breast cancer, IUD contraception equally safe in teenagers as in older women, Black men less likely to receive follow-up for elevated prostate cancer marker, PSA, More U.S. women using emergency contraception pill, No-cost contraception reduces unintended pregnancies
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT. Content is produced in accordance with fair use copyrights solely and strictly for the purpose of teaching, news and criticism. No benefit, monetary or otherwise, is realized by any participants or the owner of this domain.