Semaglutide associated with dose-dependent weight loss and resolution of nonalcoholic steatohepatitis
1. Nonalcoholic steatohepatitis (NASH) resolution without worsening of fibrosis occurred in a significantly greater percentage of patients who received semaglutide versus placebo, but the number of patients who had improvement in fibrosis without worsening of NASH was similar between groups.
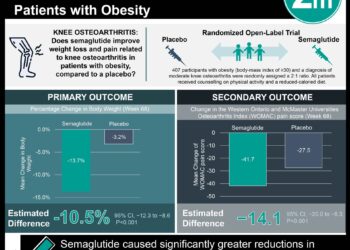
2. Patients who received semaglutide lost 8-20x more weight (depending on dose) than those who received placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Nonalcoholic steatohepatitis (NASH) is an aggressive form of fatty liver disease that is associated with fibrosis and cirrhosis as well as cardiovascular disease, chronic renal disease, and cancer. No specific pharmacotherapies currently exist for NASH, so treatment is limited to weight loss and management of coexisting conditions. Given that insulin resistance is known to be a key pathogenic driver of NASH, a number of glucagon-like peptide-1 (GLP-1) agonists are currently being investigated for their liver-specific effects. Semaglutide, one such agent, has previously been shown to facilitate reductions in body weight, transaminase levels, and various biomarkers of inflammation. In this phase 2 trial, patients were administered subcutaneous semaglutide at 3 different dosages and monitored for histological changes in liver tissue. Among patients with stage F2 or F3 fibrosis, semaglutide at all dosages resulted in a greater likelihood of NASH resolution compared to placebo. However, the percentage of these patients who experienced an improvement of at least 1 point in fibrosis stage was similar across all groups. Over twice as many patients in the 0.4-mg group achieved both of these endpoints as compared to the placebo group; conversely, worsening of fibrosis occurred in nearly twice as many patients in the placebo group versus the 0.1-mg group, which had the highest frequency of the three semaglutide groups. Further, patients in the semaglutide groups experienced greater, dose-dependent reductions in alanine- and aspartate-aminotransferase levels versus placebo. Lastly, gastrointestinal adverse events and serious adverse events were dose-independent but occurred in a greater percentage of patients in the three treatment groups versus placebo.
Click here to read the study in NEJM
Relevant Reading: Semaglutide for the treatment of non-alcoholic steatohepatitis: Trial design and comparison of non-invasive biomarkers
In-Depth [randomized controlled trial]: This double-blinded, multicenter phase 2 trial conducted from January 2017 to September 2018 involved 320 adults under 75 years of age who had a BMI >25 kg/m2, histological evidence of NASH (NAFLD activity score >4 with at least 1 point in each category: steatosis, lobular inflammation, hepatocyte ballooning), and a fibrosis stage of F1, F2, or F3. Study participants were stratified by geography (Japan vs. other), type 2 diabetes status, and fibrosis stage (F1 or F2 vs. F3) and randomly assigned in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg or corresponding placebo. Semaglutide was initiated at 0.05 mg/day, increased to 0.1 mg/day after 4 weeks, and then increased by 0.1 mg/day every 4 weeks thereafter until the target dose had been reached. After 72 weeks, a significantly greater percentage of patients with stage F2 or F3 fibrosis in all three treatment groups reached the primary endpoint—resolution of NASH (defined as no more than mild residual inflammation and no hepatocyte ballooning) with no worsening of liver fibrosis. The between-group difference compared to placebo was smaller in the 0.1-mg group (odds ratio [OR], 3.36; 95% CI, 1.29-8.86) and 0.2-mg group (OR, 2.71; 95% CI, 1.06-7.56) and largest in the 0.4-mg group (59% vs. 17%; OR, 6.87; 95% CI, 2.60-17.63; P<0.001). All four groups were similar with respect to the percentage of patients who reached the confirmatory secondary endpoint—an improvement of at least one fibrosis stage with no worsening of NASH.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![Active smoking cessation intervention may provide tangible results [Project CLIQ]](https://www.2minutemedicine.com/wp-content/uploads/2014/12/smoking-e1418644951268-350x250.jpg)