SEP-363856 reduces symptom severity for acute exacerbation of schizophrenia
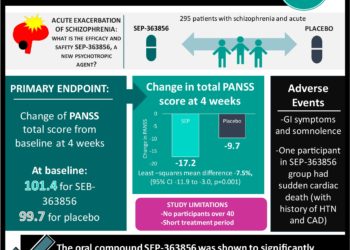
1. An oral compound, SEP-363856 was shown to significantly reduce the total score of the Positive and Negative Symptom Scale (PANSS) from baseline compare to placebo for patients with an acute exacerbation of schizophrenia.
2. One patient treated with SEP-363856 had sudden cardiac death but had history of coronary artery disease and hypertension.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Current antipsychotic treatments rely on dopamine D2 receptor antagonism for psychosis. However, the therapies are limited in the treatment of negative symptoms and cognitive impairment. SEP-363856 was developed as a new class of psychotropic agents with a non-D2-receptor binding mechanism of action for the treatment of psychosis in schizophrenia. As such, this study evaluated the efficacy and safety of SEP-363856 in patients with an acute exacerbation of schizophrenia. The participants were randomized to receive SEP-363856 or placebo treatment for four weeks. The study determined the patients in the SEP-363856 group had a lower PANSS total score from baseline compared to the placebo group. However, one patient in the treatment group did suffer a serious adverse event of sudden cardiac death.
This randomized, double-blind, trial was limited by the short treatment period, which did not allow for the longitudinal measurement of treatment effects over a six to twelve-month timer period. Another limitation of the study was patients over the age of 40 years were excluded from the trial; therefore, the trial results cannot be generalized to patients older than 40 years. Nonetheless, this study was strengthened by the even number of participants discontinuing treatment in both groups and matched sample size. For physicians, these findings highlighted an alternative therapy to be prescribed to patients suffering from an acute exacerbation of schizophrenia.
Click to read the study in NEJM
Relevant Reading: Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis
In-Depth [randomized controlled trial]: This randomized control trial enrolled 295 patients in a multicenter study from 34 sites in five countries in Eastern Europe and North America. The inclusion criteria included pubertal adolescents (12 to <18 years of age). Inclusion criteria included: patients between 18 to 40 years of age; met the criteria for schizophrenia in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition for at least six months; and had an acute exacerbation of psychotic symptoms with a duration of two months or less. The exclusion criteria for the study included three or more hospitalizations for treatment of an acute exacerbation of schizophrenia. The patients were randomized to the SEP-363856 or placebo in a 1:1 ratio. The treatment in the study began after a 14-day washout period where all previous psychotropic medications were discontinued. The primary endpoint was the change of PANSS total score, from baseline, at four weeks. The score ranged from 30 to 210, and higher scores indicated more severe psychotic symptoms. Additionally, adverse outcomes within both treatment groups were recorded. The mean total PANSS score, at baseline, was 101.4 for the SEP-363856 group and 99.7 for the placebo group. At the end of the four-week period, the least-squares means change of the total PANSS score was -17.2 in the SEP-363856 group and -9.7 in the placebo group (least-squares mean difference, -7.5, 95% confidence interval [CI], −11.9 to -3.0; p = 0.001). Gastrointestinal symptoms and somnolence were the most frequently recorded adverse events. A serious adverse event was reported for one participant in the SEP-363856 group who suffered from sudden cardiac death. The participant had a history of essential hypertension and coronary artery disease.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.