Simvastatin may slow brain atrophy in secondary progressive multiple sclerosis [MS-STAT study]
Image: PD
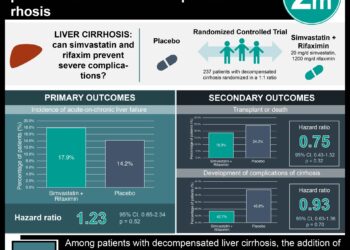
1. High dose simvastatin therapy reduced the annual brain atrophy rate over two years compared to placebo in patients with secondary progressive multiple sclerosis (MS).
2. Simvastatin was associated with a small but significant reduction in MS disability scores compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Multiple sclerosis (MS) is a major cause of disability characterized by neurological dysfunction and frequent relapses in the early stages, and a progressive functional decline with increasing brain atrophy in the absence of relapses during the advanced stages. There is currently no treatment for the advanced, secondary progressive stage of MS. Statins are widely used today for the treatment and prevention of cardiovascular disease and have been shown to have potential neuroprotective and anti-inflammatory effects. The MS-STAT trial followed 140 patients with secondary progressive MS for 24 months, with 70 patients receiving 80mg simvastatin daily and 70 patients receiving placebo. The annual brain atrophy rate was significantly lower in the simvastatin group than the placebo group, with a 43% reduction in rate. Additionally, lower MS disability scores were seen in the simvastatin group compared to the placebo group. However, there was no significant difference between the simvastatin and placebo groups in serum immunological markers, suggesting that statins may only play a neuroprotective role. This study was limited in that it was not initially designed and inadequately powered to determine the effect on disability. Further phase 3 trials are needed to confirm these promising results in the treatment of secondary progressive MS.
Click to read the study, published today in The Lancet
Relevant Reading: Secondary progressive multiple sclerosis: current knowledge and future challenges
In-Depth [randomized controlled trial]: 140 patients aged 18-65 with secondary progressive multiple sclerosis (MS) were randomly assigned to receive either 80mg of simvastatin daily (n=70) or placebo (n=70) between 2008 and 2011. Brain volume was measured by T1 MRI at 0, 12, and 25 months. Clinical outcomes were assessed at 0, 12, and 24 months and included the Expanded Disability Severity Scale (EDSS), MS functional composite scale, MS impact scale-29 (MSIS-29), and relapse frequency. Serum markers of inflammation were also measured at 0, 6, 12, and 24 months.
Whole-brain atrophy was lower in the simvastatin group at 0.288% (SD 0.521) per year than placebo group at 0.584% (SD 0.498), with an adjusted difference in atrophy rate of -0.254% per year (95% Confidence Interval [CI], -0.422 to -0.087; p=0.003). The simvastatin group also had lower disability scores in two of three outcomes at 24 months compared to placebo, with a significant difference in the EDSS (difference -0.254; 95% CI -0.464 to -0.069; p<0.01) and MSIS-29 (-4.78; 95% CI -9.39 to -0.02; p<0.05). There was no significant difference between the simvastatin and placebo group in the rate of new and enlarging lesions, as well as in the reduction of IFN-γ, IL-4, IL-10, and IL-17 expression on T cells at any time point.
More from this author: Ruptured abdominal aortic aneurysm mortality greater in England vs. USA, Diffusion-weighted MRI shows promise as a radiation-free alternative for pediatric tumor staging
©2012-2014 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.