The addition of liraglutide to diet and exercise leads to increased weight loss
1. For overweight and obese patients, the addition of liraglutide once daily to a diet and exercise regimen resulted in increased weight loss and improved overall health.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Obesity is an epidemic currently sweeping across the nation and worldwide. It is well documented that for overweight and obese patients, weight loss reduces morbidity and mortality. However, weight loss is often difficult to achieve and, more importantly, to maintain. Numerous agents and procedures have been developed and tested to help patients achieve weight loss. Bariatric surgery remains the most effect weight loss method. Recent drug trials have focused on glucagon-like peptide-1 (GLP-1) mimetics. These agents are theorized to work like endogenous GLP-1 in stimulating insulin secretion, lowering postprandial glucagon levels, slowing gastric emptying, and reducing appetite.
This study found that when liraglutide, a GLP-1 analogue, was added to a diet and exercise regimen in obese patients, greater weight loss was achieved. In addition, patients given liraglutide also attained statistically significant improvements in glycemic control, blood pressure, and quality of life scores. One limitation of this study is its lack of inclusion of diabetic patients. As a majority of overweight patients are often also diabetic, addition studies are needed to evaluate the effect of the drug in this population. Patients in this study were followed for a total of 56 weeks. Longer term follow up would be needed to assess for maintenance of weight loss.
Click to read the study, published today in NEJM
Click to read an accompanying editorial in NEJM
Relevant Reading: Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials
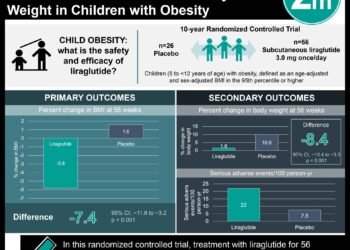
In-Depth [randomized controlled trial]: This trial conducted at 191 sites in 27 countries randomized 3731 patients to life style intervention plus liraglutide or lifestyle intervention plus placebo. Patients in the liraglutide group received once-daily subcutaneous injections of liraglutide, starting at a dose of 0.6 mg with weekly 0.6-mg increments to 3.0 mg. Both groups were provided counseling on lifestyle modification. At the end of 56 weeks, patients in the liraglutide group lost an average of 8.0±6.7% (8.4±7.3 kg) of their body weight as compared to patients in the placebo group who had lost an average of 2.6±5.7% (2.8±6.5 kg). In addition, a greater reduction in glycated hemoglobin, fasting glucose, and fasting insulin levels were observed in the liraglutide group than in the placebo group. The liraglutide group also scored higher on the SF-36 for overall physical and mental health as well as the Impact of Weight on Quality of Life questionnaire when compared to the placebo group.
The most common side effects experienced by patients in the liraglutide group were associated with the gastrointestinal system. Gastrointestinal events were also the most common reason that patients receiving liraglutide withdrew from the trial. Additionally, more cases of cholelithiasis and cholecystitis occurred in the liraglutide group.
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![Type I diabetes not associated with early menopause [OVADIA study]](https://www.2minutemedicine.com/wp-content/uploads/2014/12/diabetes1_edited-350x250.jpg)