The CLL14 trial: longer progression-free survival for CLL patients with coexisting conditions treated with venetoclax
1. In untreated chronic lymphocytic leukemia (CLL) patients with coexisting conditions, patients treated with venetoclax–obinutuzumab experienced greater progression-free survival compared to patients treated with chlorambucil–obinutuzumab.
2. Rates of adverse events in the two treatment groups were similar.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Many patients with CLL are elderly and have clinically relevant comorbid health conditions. The BCL2 antiapoptotic protein is overexpressed in CLL, and venetoclax is an oral BCL2 homology domain 3 mimetic compound which aids to induce apoptosis in B-cells. Whether venetoclax can improve treatment efficacy in CLL patients with comorbidities while limiting toxicity is undetermined. This open-label, phase 3 trial showed progression-free survival is significantly increased in patients taking venetoclax as part of their treatment regimen. Adverse outcomes compared to the current standard treatment regimen were similar.
This study supports the use of venetoclax in CLL patients with comorbidities as it optimizes a key survival outcome while toxicity is comparable with the current treatment standard. The study is limited in its strength to detect a difference in overall survival.
Click to read the study, published today in NEJM
Relevant Reading: Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia
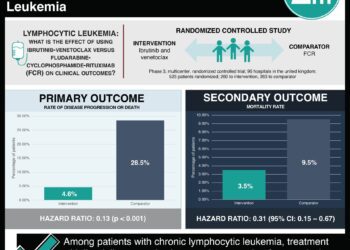
In-Depth [randomized controlled trial]: This phase 3, open-label, randomized controlled trial enrolled patients in 21 countries. Eligible patients had untreated CD20+ CLL and a Cumulative Illness Rating Scale score of greater than 6. Patients were randomized in a 1:1 fashion to either venetoclax–obinutuzumab (n=216) or chlorambucil–obinutuzumab (n=216) groups. Patients in both groups were treated for 12 cycles lasting 28 days each. Venetoclax dosage was ramped-up over 5 weeks until the full treatment dosage was reached. The primary end point of progression-free survival at 24 months showed a higher rate in the venetoclax group (88.2%; 95% confidence interval [CI], 83.7 to 92.6) compared to the chlorambucil group (64.1%; 95% CI, 57.4 to 70.8). At a medial follow-up of 28.1 months, 30 and 77 primary end point events had been reached in the venetoclax and chlorambucil groups, respectfully (hazard ratio, 0.35; 95% CI, 0.23 to 0.53; P<0.001). An increased progression-free survival in the venetoclax group was seen in patients with a TP53 deletion or mutation. At 3 months after treatment completion, more venetoclax treated patients were negative for minimal disease residue in either the blood or the bone marrow. Patients with any degree of treatment response was also higher in the venetoclax group (84.7% vs. 71.3%, P<0.001), and significantly more patients also experienced complete response in the venetoclax group (49.5% vs. 23.1%, P<0.001). At least one adverse event was reported in greater than 94% of participants in either group. Adverse events leading to treatment discontinuation occurred in approximately 16% of patients in both groups. Tumor lysis syndrome and fatal adverse events also occurred at similar rates between the two groups.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc