The EINSTEIN-PE trial: Rivaroxaban to treat pulmonary embolism [Classics Series]
Image: PD
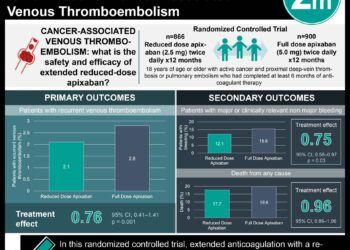
1. Rivaroxaban, an oral factor Xa inhibitor, was non-inferior to standard anticoagulation therapy (i.e. low-molecular weight heparin and a vitamin K antagonist) in the prevention of recurrent thromboembolism following a pulmonary embolism.
2. The rate of major bleeding was significantly lower with rivaroxaban than with standard anticoagulant therapy.
Original Date of Publication: April 5, 2012
Study Rundown: For many years, standard therapy for pulmonary embolism (PE) has consisted of low-molecular weight heparin (LMWH) followed by a vitamin K antagonist (VKA), such as warfarin. Although effective, treatment with warfarin requires frequent blood tests and is thus burdensome for patients. In summary, the EINSTEIN-PE study demonstrated that rivaroxaban, an oral factor Xa inhibitor, can be administered without laboratory monitoring in the prevention of recurrent thromboembolism following an acute PE. Specifically, treatment with rivaroxaban alone was non-inferior to standard therapy in preventing recurrent thromboembolism (HR 1.12; 95%CI 0.75-1.68). Rivaroxaban use was also linked with significantly fewer major bleeding events when compared with standard therapy.
A major limitation of the EINSTEIN-PE trial was its open-label design, as this increases the risk of bias. Notably, 5% of patients participating in the study had cancer. Previous studies, including the CLOT trial, have helped establish guidelines recommending the use of LMWH in treating thromboembolism in the context of malignancy, and this recommendation has not changed in light of the findings of the EINSTEIN-PE trial. The study was funded by Bayer HealthCare and Janssen Pharmaceuticals.
Please click to read study in NEJM
In-Depth [randomized, controlled study]: Originally published in 2012, the EINSTEIN-PE trial was a randomized, open-label, non-inferiority trial involving 4,832 patients. Eligible patients were those who had an acute, symptomatic PE with or without symptomatic deep-vein thrombosis (DVT). Patients were randomized to receive rivaroxaban, or standard therapy consisting of enoxaparin with either warfarin or acenocoumarol. The primary efficacy outcome was recurrent venous thromboembolism (i.e., PE and/or DVT), and the primary safety outcome was clinically relevant nonmajor bleeding or major bleeding (defined as bleeding in critical sites). Rivaroxaban was non-inferior to standard therapy in preventing recurrent venous thromboembolism post-PE (HR 1.12; 95%CI 0.75-1.68). The rate of major bleeding was also lower in the rivaroxaban group, as compared to standard therapy (HR 0.49; 95%CI 0.31-0.79).
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.