The PAGE trial: clinical outcomes do not improve with inhaled GM-CSF in pulmonary alveolar proteinosis patients
1. In patients with pulmonary alveolar proteinosis, use of twice daily inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) did not improve clinical outcomes of vital capacity or functional walk test compared to placebo treated patients.
2. Laboratory and imaging metrics suggestive of reduced pulmonary disease were noted in GM-CSF treated patients.
Evidence Rating Level: 1 (Excellent)
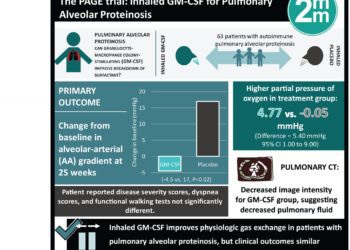
Study Rundown: Accumulation of surfactant within pulmonary alveoli, often due to autoimmunity against GM-CSF, is the pathologic finding observed in patients with pulmonary alveolar proteinosis. The present treatment to reduce surfactant is lung lavage which is invasive and requires repeated procedures. Preclinical and early clinical studies have shown inhaled GM-CSF can break down surfactant and improve gas exchange, though the efficacy of treatment in patients with mild-to-moderate disease is unknown. The Pulmonary Alveolar Proteinosis GM-CSF Inhalation Efficacy (PAGE) trial randomized patients to receive inhaled GM-CSF or placebo over 24 weeks. The primary outcome of change in alveolar-arterial (AA) gradient between baseline and week 25 showed a significant improvement in the treatment group. Additionally, reduced pulmonary intensity characteristic of reduced fluid was seen in treated patients. Clinical metrics including patient-reported disease outcomes, vital capacity, and functional walk testing were similar in the treatment and placebo groups.
This randomized trial suggests inhaled GM-CSF is improving physiologic gas exchange in pulmonary alveolar proteinosis patient lungs. Validation of clinical efficacy may require longer follow-up, alternate dosing, or study in patients with a different disease severity.
Click to read the study in NEJM
Relevant Reading: Prevalence and healthcare burden of pulmonary alveolar proteinosis
In-Depth [randomized controlled trial]: This multicenter, randomized, placebo-controlled study enrolled patients in 2016. Eligible adult patients had a diagnosis of autoimmune pulmonary alveolar proteinosis, clinical symptoms of pulmonary disease, and a reduced partial pressure of oxygen. Patients who had a recent lung lavage procedure, had previously received GM-CSF, or had markedly decreased oxygenation measurements were excluded. Patients were randomized to receive 125 μg of GM-CSF twice daily (n=33), with alternating weeks of taking the treatment or not, or a placebo for a total intervention period of 24 weeks (n=30). The primary endpoint of change from baseline in AA gradient at 25 weeks was improved in the GM-CSF group compared to placebo patients (mean change from baseline, −4.50 mmHg vs. 0.17 mmHg; P=0.02). Similarly, a higher partial pressure of oxygen was observed in the treatment group (4.77mm Hg vs. -0.05 mmHg for placebo; difference vs. placebo, 5.40; 95% confidence interval [CI], 1.00 to 9.90). Pulmonary computed tomography imaging showed significantly decreased image intensity in treated patients after 24 weeks, suggesting reduced pulmonary fluid characteristic of disease pathology. Patient reported pulmonary disease severity scores, dyspnea scores, and functional walking test metrics did not differ between treated and placebo patients. In subgroup analysis of smokers, AA gradients were not significantly affected with GM-CSF treatment. Occurrence of adverse events was similar between treated and placebo patients.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc