TICO Trial: Ticagrelor monotherapy vs. extended dual anti-platelet therapy after placement of drug-eluting stents resulted in a reduction in adverse major bleeding events
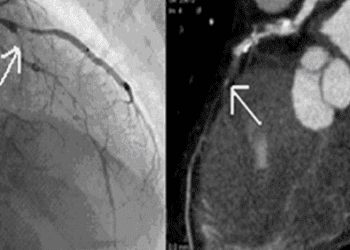
1. Patients status-post placement of drug-eluting stents (DES) for acute coronary syndrome received three months of Ticagrelor and Aspirin dual-antiplatelet therapy (DAPT), followed by nine months of DAPT or Ticagrelor monotherapy.
2. At one year, patients who received Ticagrelor alone experienced significantly fewer adverse major bleeding events. There was no reduction in adverse cardiac or cerebrovascular events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: In an effort to determine whether Ticagrelor monotherapy, as compared to a standard 1-year of DAPT following DES placement reduces the risk of adverse events, a randomized multi-center trial was conducted across 38 hospitals in South Korea. After DES placement, patients received three months of DAPT treatment, followed by either Ticagrelor monotherapy or continued treatment with DAPT, for one year of total treatment. Of the patients who completed the study, 1339 received Ticagrelor alone and 1321 received DAPT. The mean age was 61, and 80% were men. Patients predisposed to major bleeding events (recent traumatic brain injury, history of hemorrhagic stroke, etc.), were excluded from this study. After one year of total treatment, patients who received Ticagrelor monotherapy experienced significantly fewer major bleeding events than those receiving DAPT. However, there was no significant different in adverse cardiac, cerebrovascular events or deaths between the two groups. Despite the lack of statistical significance in regard to the risk of cardiac or cerebrovascular events, this data suggests that following short-term administration of DAPT, monotherapy with an anti-platelet agent such as Ticagrelor has the potential to be a safer strategy than extended DAPT. However, this study was not blinded and would benefit by expansion to include a more geographically, racially, and gender diverse patient population.
Click to read the study, published today in JAMA
In-Depth [randomized controlled trial]: Following acute coronary syndromes, patients commonly undergo placement of DES, followed by a year of DAPT. While DAPT is fundamental in preventing dangerous clotting around these patients’ new stents, it is also known to be associated with some adverse side effects, such as increased risk of bleeding. This study was conducted in an attempt to determine whether the discontinuation of aspirin after three months of DAPT would reduce associated major adverse events.
Patients who received a DES following a major cardiac event (non-ST or ST-elevated myocardial infarction, or unstable angina) were included in this study. All of these patients received DAPT consisting of aspirin (180 mg/day) and Ticagrelor (90 mg/ bid) for three months. Patients were then divided into groups of either Ticagrelor monotherapy or continued DAPT for a further nine months. Net adverse events within this year of treatment were recorded.
Within one year of stent placement, 3.9% of patients receiving monotherapy experienced an adverse event, as compared to 5.9% in the DAPT group. Patients receiving DAPT experienced a significantly higher rate of major bleeding events (3%) than those on monotherapy (1.7%). However, the differences between the rate of adverse cardiac and cerebrovascular events or death between the two groups were not significantly significant (HR 0.69, HR 0.58, HR 0.70, respectively).
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.