Tirbanibulin ointment potentially effective for actinic keratosis treatment
1. In these two identical, randomized, placebo-controlled clinical trials, tirbanibulin ointment was more effective than placebo in reducing the actinic keratosis.
2. Nearly half of the patients who had complete resolution of the lesion had a recurrence at one-year.
Evidence Rating Level: 1 (Excellent)
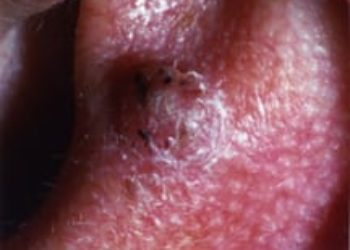
Study Rundown: Actinic keratosis is a common pre-malignant lesion of the skin with possible progression to cutaneous squamous cell carcinoma. Tirbanibulin is an inhibitor of tubulin polymerization inhibitor and Src kinase activity and had shown preliminary effectiveness. In these two identical randomized, placebo-controlled clinical trial, adults with actinic keratosis were assigned either tirbanibulin ointment for five days or vehicle ointment (placebo) to the lesion area. The study determined a superior amount of complete clearance in both trials. Furthermore, the percentage of patients with partial clearance was higher in the treatment group compared to the placebo. However, one year after treatment, the recurrence of actinic keratosis was nearly half in the complete resolution group. A limitation of the trial design is the possible open-label nature of the trial as the investigators may have suspected the treatment based on adverse reactions. In general, these trials showed tirbanibulin topical ointment is a potential treatment option for actinic keratosis.
Click here to read the study in the NEJM
Relevant Reading: Management of actinic keratosis at specific body sites in patients at high risk of carcinoma lesions: expert consensus from the AKTeam™ of expert clinicians
In-Depth [randomized controlled trial]: The two phase 3, placebo-controlled, randomized trials of 702 participants with untreated facial or scalp actinic keratosis. Participants were randomized 1:1 to either topical tirbanibulin or vehicle ointment in an area containing four to eight lesions once daily for five days. The primary endpoint was the percentage of patients with a complete reduction (100% reduction in lesions) in the lesions in the applied area by day 57. Secondary endpoints included the percentage of patients with a partial reduction (≥ 75%) at day 57. In the first trial, the primary endpoint was met in 44% of treatment participants compared to 5% of placebo participants. In the second trial, the primary endpoint was met in 54% of treatment participants compared to 13% of placebo participants. Across the two trials, complete response occurred in 49% of treatment participants compared to 9% placebo participants (difference, 41%; 95% confidence interval [CI] 35 to 47). The pooled secondary endpoint was met in 72% of treatment participants compared to 18% placebo participants (difference, 54%; 95% CI, 48-60%). At one year follow-up for patients with complete resolution, 58% of patients had recurrent lesions and 52% had new lesions only. Local reactions included erythema (91%) and flaking or scaling (82%). Adverse events included application-site pain (10%) and application-site pruritis (9%). Local reactions and adverse events resolved spontaneously and most were mild.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.