Transcatheter aortic-valve replacement noninferior to surgery in intermediate-risk patients: The PARTNER 2 study
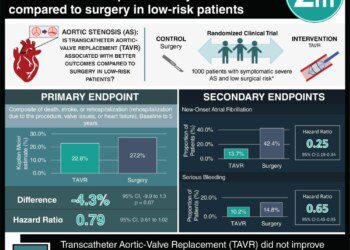
1. For intermediate-risk surgical candidates with aortic stenosis, transcatheter aortic-valve replacement (TAVR) was non-inferior to surgical aortic-valve replacement based on all-cause death and disabling stroke at 2 years, as well as 30-day clinical endpoints.
2. TAVR had better aortic valve area and gradient compared to surgery based on echocardiographic findings, but a higher incidence of paravalvular aortic regurgitation.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Aortic stenosis, characterized by a long period of latency followed by a rapidly progressing symptomatic stage, carries high mortality if left untreated. Since aortic valve replacement is the only definitive therapy, TAVR was developed as a less invasive way to insert a bioprosthetic valve in patients who are considered unsuitable for surgery. In these high-risk patients, TAVR has been established as superior to other therapeutic options; however, TAVR had not been previously compared to surgery.
This multicenter randomized controlled trial (PARTNER 2) compared outcomes of TAVR and surgery for intermediate-risk patients. Based on the results, TAVR appears to be at least as effective as surgery for treating aortic stenosis in intermediate-risk patients at the 2-year follow-up. The study showed no difference in all-cause death or disabling stroke at 2 years. Furthermore, the transfemoral cohort had a significantly lower all-cause death rate and disabling stroke than surgery. Of the 30 day clinical outcomes major vascular complications were more frequent in the TAVR group compared to the surgery group. TAVR showed a significant increase in aortic valve area and decrease in aortic-valve gradients; however, paravalvular regurgitation was significantly higher in the TAVR cohort. A significantly higher number of TAVR patients were noted to have mild regurgitation at 2 years. Longer follow-up may be needed to assess further progression and the durability of TAVR.
Click to read the study, published today in NEJM
Relevant Reading: Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery
In-Depth [randomized controlled trial]: This PARTNER 2 trial enrolled 2032 patients randomized to either TAVR (n=1011) or surgery (n=1021). Within the TAVR cohort, 236 patients (23.3%) were assigned transthoracic placement and the remaining to transfermoral placement. All patients were assessed with validated criteria from the Society of Thoracic Surgeons as intermediate-risk for surgery. The primary end point was all-cause death or disabling stroke at 2 years. Short-term clinical end points were assessed at 30 days and included major vascular complications, life-threatening bleeding, acute kidney injury, new-onset atrial fibrillation, need for new pacemakers, and endocarditis.
No difference in all-cause death or disabling stroke was found between TAVR and surgery at 2 years (risk ratio = 0.92, p<0.001), meeting the criterion for noninferiority. The transfemoral cohort had a lower all-cause death rate and disabling stroke than surgery (HR in the intention-to-treat analysis, 0.79; 95% [CI], 0.62 to 1.00; p=0.05). Of the 30 day clinical outcomes, only major vascular complications were more frequent in TAVR compared to surgery (TAVR 7.9% vs. surgery 5.0%, p=0.008). Life-threatening bleeding (10.4% vs. 43.4%, p<0.001), acute kidney injury (1.3% vs. 3.1%, p=0.006), and new-onset atrial fibrillation (9.1% vs. 26.4%, p<0.001) were all less frequent in TAVR. Repeat hospitalization at 2 years, need for new pacemakers, and endocarditis were all similar between the two cohorts.
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.