Typhoid vaccine Phase 3 trial in Nepal
1. In Nepal, the typhoid conjugate vaccine has proven to be very effective in protecting against typhoid fever.
2. The typhoid antibody titers more than quadrupled in the participants that received the typhoid conjugate vaccine.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Typhoid fever is a bacterial infection caused by Salmonella that leads to a high fever, weakness, stomach pain, and rash. It is a major public health problem in the summer months among children in South Asian countries, such as India, Bangladesh, Pakistan, and Nepal. The oral vaccine has proven to be less effective in children than adults for multiple reasons. This randomized controlled trial was phase 3 of the typhoid conjugate vaccine to determine efficacy in Nepal, a typhoid fever endemic area. Children in this study received either the typhoid vaccine or control vaccine and were followed for 6 months. In participants who received the typhoid vaccine, typhoid fever occurred less commonly, and antibody titers were higher than the control group. Within a week of vaccination, the most common side effects were pain at the injection site, fever, vomiting, and diarrhea. The typhoid vaccine group reported less adverse events within 1 month and 6 months of the vaccine, when compared to the control group. No deaths related to the vaccine were reported.
Follow-up of these participants is on-going and is important to determine long-term efficacy and safety of the vaccine. If this vaccine proves to be effective long-term, it will be able to help prevent typhoid fever among children and travelers to high-risk countries.
Click to read the study in NEJM
Relevant Reading: The Severe Typhoid Fever in Africa Program Highlights the Need for Broad Deployment of Typhoid Conjugate Vaccines
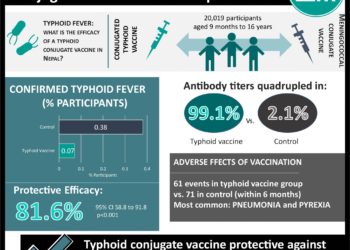
In-Depth [randomized controlled trial]: This phase 3, double-blind, randomized controlled trial included 20,019 participants from Nepal to examine the effectiveness of the typhoid conjugate vaccine in an endemic area. Participants received the vaccine between 2017 and 2018, with follow-up into 2019. Children between the ages of 9 months and 16 years in good health were included in the study. Children were randomly assigned to groups based on age – either the conjugated typhoid vaccine group or meningococcal conjugate vaccine (control). Participants were followed to determine if the vaccine prevented typhoid fever, developed immunogenicity, and reported adverse events. Confirmed typhoid fever occurred in 0.07% of the typhoid vaccine group, compared to 0.38% of the control group. The protective efficacy of the typhoid vaccine was 81.6% (95% CI, 58.8 to 91.8; P<0.001). Vaccine efficacy was 85.1% in participants who had at least 3 days of fever (95% CI; 49.7 to 95.6). 28 days after receiving the vaccine, antibody titers more than quadrupled in 99.1% of the typhoid vaccine group versus 2.1% in the control group. Within 7 days of vaccination, the most common side effects were pain at vaccination site, feeling generally unwell, having a fever, vomiting and diarrhea, and decreased appetite. Within 28 days of the vaccination, 7 adverse events were reported in the typhoid vaccine group versus 10 in the control group. Within 6 months of the vaccination, 61 adverse events were reported in the typhoid vaccine group versus 71 in the control group. The most common adverse events reported were pneumonia and pyrexia.
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.