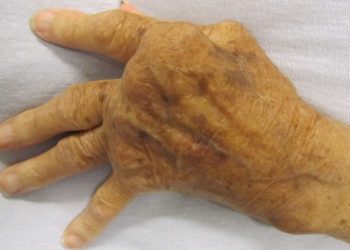
Upadacitinib may be superior to abatecept for refractory rheumatoid arthritis
1. Upadacitinib was superior to abatacept in achieving remission at 12 weeks for patients with rheumatoid arthritis refractory to biologic disease-modifying antirheumatic drugs (DMARDs).
2. Upadacitinib was associated with more severe adverse events compared to abatacept.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Abatacept treats rheumatoid arthritis by inhibiting T-cell proliferation and B-cell stimulation by binding to CD80 and CD86 receptors on antigen-presenting cells. Abatacept is a type of conventional synthetic disease-modifying antirheumatic drug (DMARD). In cases DMARD treatment failure occurs, upadacitinib – a Janus kinase (JAK) inhibitor – can be used to achieve disease remission. Though upadacitinib has been shown to be able to treat rheumatoid arthritis, the efficacy and safety of the treatment compared to abatacept has yet to be determined. As such, this study compared the efficacy and safety of upadacitinib to abatacept in patients with inadequate response to biologic DMARD. The study results demonstrated upadacitinib was superior to abatacept in achieving disease remission at 12 weeks based on the Disease Activity Score for 28 Joints (DAS28-CRP). The randomized trial was limited by the use of the DAS28-CRP. The subjectivity of the scoring system introduced variability between the scoring of patients and their perspective of disease activity. Nonetheless, this study’s results are significant, and its findings highlight the benefit of a therapy after previous unsuccessful treatments for rheumatoid arthritis remission.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This randomized control trial enrolled 613 participants in a multicenter study at 120 sites in 28 countries. Participants included in the study had a rheumatoid arthritis diagnosis for at least 3 months, moderate-to-severe disease, and unsuccessful DMARD treatment for at least 3 months. Participants with previous exposure to JAK inhibitor or abatacept were excluded from this study. The participants were randomized in a 1:1 ratio to receive oral upadacitinib or intravenous abatacept, respectively. The primary end point was change in the DAS28-CRP from baseline at 12 weeks and percentage of participants with clinical remission. Clinical remission was defined as a DAS28-CRP score of less than 2.6. The mean DAS28-CRP, at baseline, was 5.70 in the upadacitinib group compared to 5.88 in the abatacept group. At week 12, the mean score change from baseline was -2.52 points in the upadacitinib group compared to -2.00 points in the abatacept group (difference, -0.52 points; 95% confidence interval [CI], -0.69 to -0.35; P<0.001 for superiority). Furthermore, the percentage of patients in remission according to the DAS28-CRP score was 30.0% with upadacitinib compared to 13.3% with abatacept (difference, 16.8 percentage points, 95% CI, 10.4 to 23.2; P<0.001). At the end of the trial, serious adverse events were numerically higher in the upadacitinib group (10 participants, 3.3%) compared to the abatacept group (5 participants, 1.6%). Opportunistic infections were reported in 4 participants within the upadacitinib group compared to 1 participant in the abatacept group. Additionally, 23 participants (7.6%) in the upadacitinib group experienced hepatic disorders compared 5 participants (1.6%) in the abatacept group. Taken together, upadacitinib was superior to achieving remission in participants with refractory disease compared to abatacept; however, upadacitinib was associated with more serious adverse events.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.