Vaginal misoprostol faster-acting, less painful than buccal
1. Vaginal misoprostol resulted in faster induction and completion of second-trimester terminations than buccal administration.
2. Expected and perceived pain scores were higher in the buccal group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Approximately 8 percent of the 1.2 million terminations performed in the United States each year occur during the second trimester of pregnancy. These procedures are performed for various reasons, including treatment of intrauterine fetal demise and septic abortion, fetal anomalies incompatible with life as well as morbid maternal conditions necessitating pregnancy termination (e.g. advanced-stage cancer diagnoses, severe and life-threatening pre-eclampsia remote from viability). The majority of these procedures are performed surgically (95%), through a dilation and evacuation (D&E) procedure. But with modern dosing regimens, medical terminations, which involve inducing preterm labor through the use of medications like mifepristone and misoprostol, have been shown to be safe and effective with overall complication rates of <1%. In fact, in many European countries, medical treatment is the standard of care for second trimester pregnancy terminations. Misoprostol can be administered orally, vaginally and buccally. Studies have previously found vaginal misoprostol is more effective than oral misoprostol. However few studies have directly compared vaginal with buccal administration. In the present study, researchers randomized women undergoing second-trimester pregnancy termination to receive equal dosing regimens of buccal or vaginal misoprostol.
Vaginal misoprostol was equally as effective as buccal misoprostol, and resulted in a significantly shorter induction-to-termination interval. Strengths included randomized design and use of the most common misoprostol-dosing regimen, permitting comparability of this study with others. Complex patients—such as those with intrauterine fetal demise, premature rupture of membranes (PROM), and those with uterine scars—were excluded from the study, which precludes applicability of study findings to these populations. Future studies might also assess for side effects (nausea, cramping, vomiting) and patient satisfaction associated with various routes of administration to better care for this vulnerable population of women.
Click to read the study in Obstetrics & Gynecology
Relevant Reading: A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination
In-Depth [randomized controlled trial]: One-hundred and thirty women seeking termination of a viable second-trimester (13-24 weeks) pregnancy were randomized to receive either vaginal or buccal misoprostol. Misoprostol was dosed in 400 μg, every 3 hours, up to 6 doses in 24 hours, for up to 48 hours, or until delivery took place. Primary outcome was induction-to-termination time interval. Secondary outcomes included pain, rate of complete abortion, number of misoprostol doses, side effects, maternal complications, and change in hemoglobin.
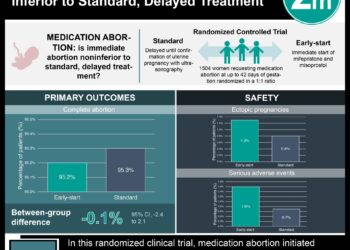
Compared to those randomized to buccal administration, women who received vaginal misoprostol had shorter induction-to-termination intervals (25 vs. 40 hours, p=0.001) and a higher completion rate at 48 hours (91% vs. 68%, p=0.001), though overall completion rates were similar between groups. Expected and perceived pain scores were higher in the buccal group.
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.