#VisualAbstract: Abiraterone acetate improves survival in high-risk non-metastatic prostate cancer compared to control
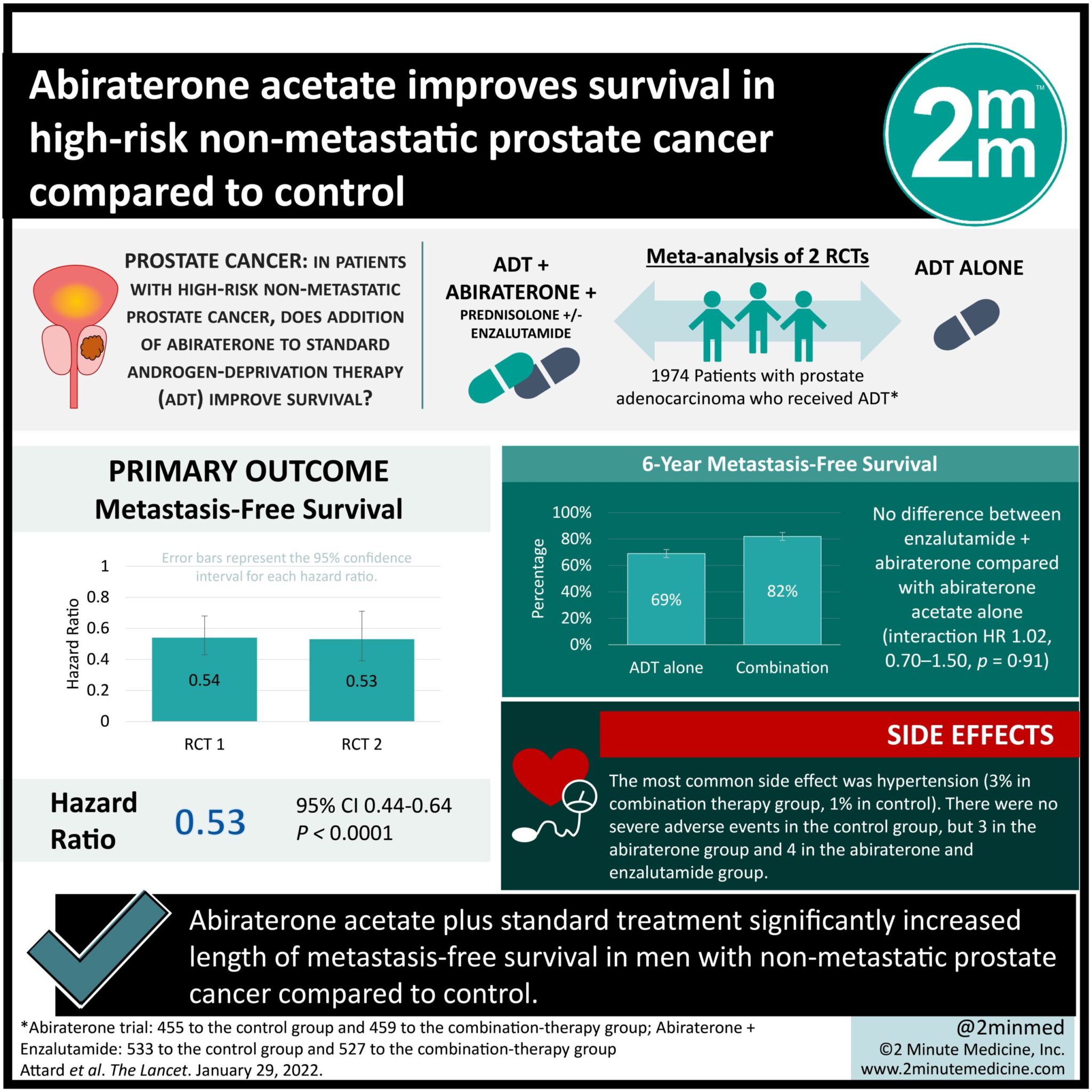
1. Abiraterone acetate plus standard treatment significantly increased length of metastasis-free survival in men with non-metastatic prostate cancer compared to control.
2. Regarding adverse events, hypertension was more common in the treatment group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The 5-year survival of non-metastatic prostate cancer is almost 100%; however, this drops to 30% once the cancer has metastasized. Thus, it is desirable for this condition to be treated prior to metastasizing. This meta-analysis compiled the results from two previous randomized controlled trials (RCTs) comparing abiraterone plus standard treatment (androgen-deprivation therapy, ADT) versus ADT alone. The results of this study found that in men with non-metastatic prostate cancer, combination treatment with ADT and abiraterone significantly reduced rates of metastases. Metastasis-free, progression-free, and overall survival were all significantly improved in the abiraterone group compared to control. Abiraterone was also well tolerated, with the most common side effect being hypertension. Serious adverse events were rare. Limitations of this study include the open-label design of the included trials. Nonetheless, this study provides high-quality evidence from two RCTs that support the use of abiraterone as standard treatment for men with non-metastatic prostate cancer.
Click to read the study in the Lancet
Relevant Reading: Diagnostic characteristics of lethal prostate cancer
In-Depth [meta-analysis]: This meta-analysis was of two previous RCTs. The two studies had the same eligibility criteria, which included prostate adenocarcinoma confirmed histologically, and no overlapping control participants. All patients received androgen-deprivation therapy (ADT) the current standard treatment. Patients were randomly assigned 1:1 to receive either only ADT (“control”) or combination therapy treatment with abiraterone and prednisolone with or without enzalutamide. Patients were also separated based on non-metastatic or metastatic disease. Final treatment numbers were as follows: non-metastatic control (n = 455), non-metastatic abiraterone (n = 451), non-metastatic control #2 (n = 533), non-metastatic abiraterone and enzalutamide (n = 527), metastatic (n = 1919). The decision was made to combine the non-metastatic groups into either control or combination treatment, as there was no significant difference seen between abiraterone alone versus abiraterone and enzalutamide.
The median follow-up was 72 months. The primary outcome was metastasis-free survival, which was significantly improved in the combination treatment groups (HR 0.53, 95% CI 0.44-0.64, p<0.0001) compared to the control group of ADT alone. 6-year metastasis-free survival was 69% (66-72) in the control group versus 82% (79-85) in the treatment group. A similar result was seen for overall survival rates (p<0.0001) and progression-free survival (p<0.0001). The most common treatment-related side effect was hypertension (5% of abiraterone group, 1% of control). There were no severe adverse events in the control group, but 3 in the abiraterone group and 4 in the abiraterone and enzalutamide group.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.