#VisualAbstract: Budesonide may be effective for slowing renal function decline in patients with IgA nephropathy
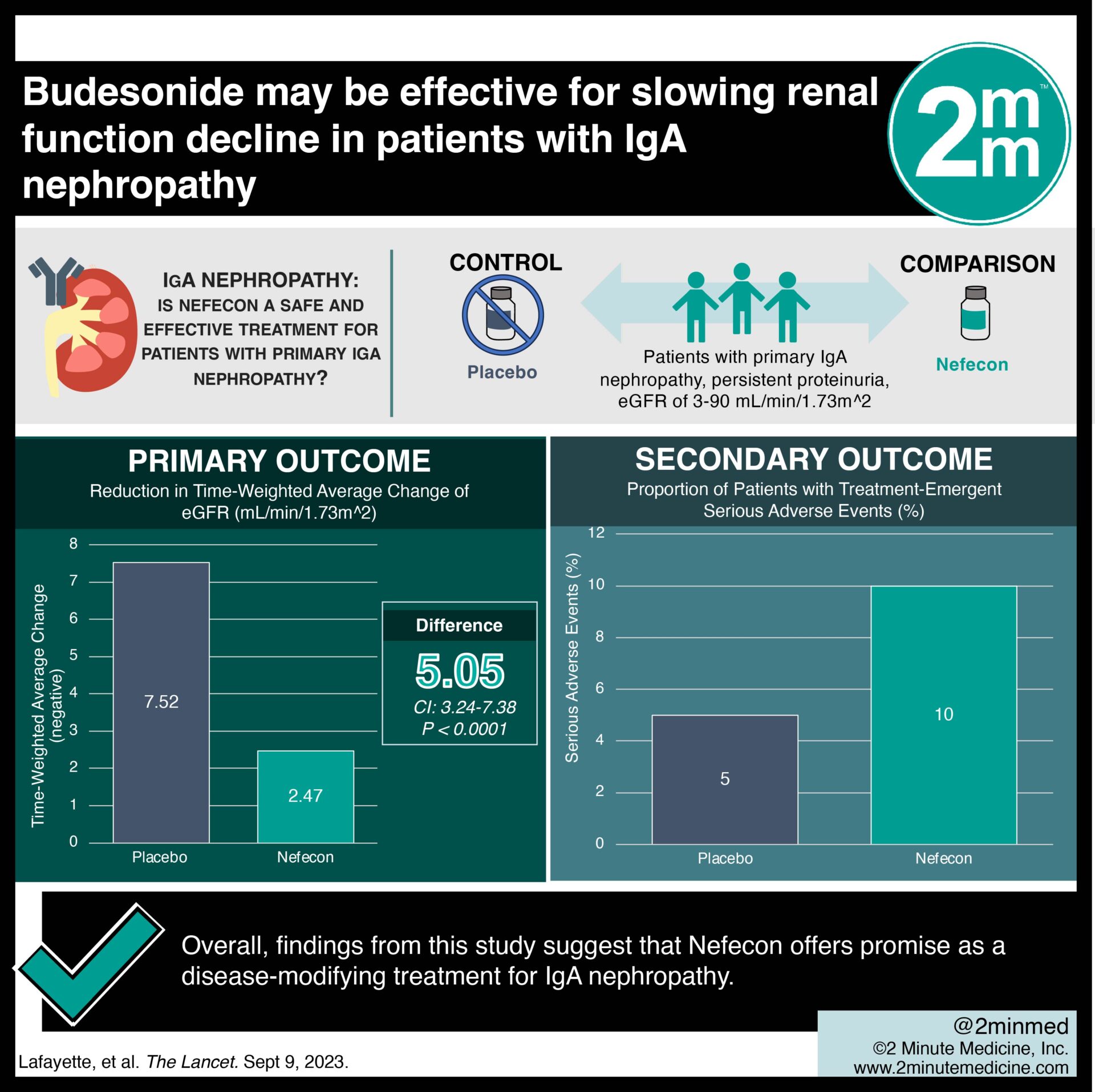 1. Reduction in time-weighted glomerular filtration rate (GFR) decline was significantly greater in Nefecon versus placebo.
1. Reduction in time-weighted glomerular filtration rate (GFR) decline was significantly greater in Nefecon versus placebo.
2. Majority of adverse events were mild-to-moderate in nature with no treatment-related deaths.
Evidence Rating Level: 1 (Excellent)
Study Rundown: IgA nephropathy is a chronic immune-mediated kidney disease with marked proteinuria. Nefecon, a novel, targeted-release form of budesonide, is designed to inhibit galactose-deficient IgA1 (gd-IgA1) within the gastrointestinal tract, although little is currently known. This randomized controlled trial aimed to assess the safety and efficacy of Nefecon in patients with primary IgA nephropathy. Adult patients with primary IgA nephropathy who exhibited persistent proteinuria despite optimal therapy were randomly assigned to receive either Nefecon or placebo for 9 months, followed by a 15-month observation period. The primary outcome was the time-weighted average of eGFR while the key secondary outcome was treatment-emergent adverse events. According to study results, 9-month treatment with Nefecon resulted in a reduction in both GFR decline and proteinuria versus placebo. Although this study was well done, it was limited by a short follow-up period, thus affecting the validity of its findings.
Click to read the study in The Lancet
Relevant Reading: Intensive Supportive Care plus Immunosuppression in IgA Nephropathy
In-depth [randomized-controlled trial]: Between Sept 5, 2018, and Jan 20, 2021, 364 patients were screened for eligibility across 132 hospital-based clinical sites in 20 countries. Included were patients ≥ 18 years old with primary IgA nephropathy, eGFR of 35-90 mL/min/1.73 m^2, and persistent proteinuria (defined by urine protein–creatinine ratio ≥ 0.8 g/g or proteinuria ≥ 1 g/24 h). Altogether, 364 patients (182 each in Nefecon and placebo) were included in the final analysis. The primary outcome of a reduction in time-weighted eGFR decline was significantly greater in Nefecon versus placebo (difference 5.05 mL/min/1.73 m^2, 95% confidence interval [CI] 3.24-7.38, p<0.0001). Similarly, the secondary outcome revealed a favourable safety profile for Nefecon with well-tolerated treatment-emergent adverse events. Overall, findings from this study suggest that Nefecon offers promise as a disease-modifying treatment for IgA nephropathy.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.