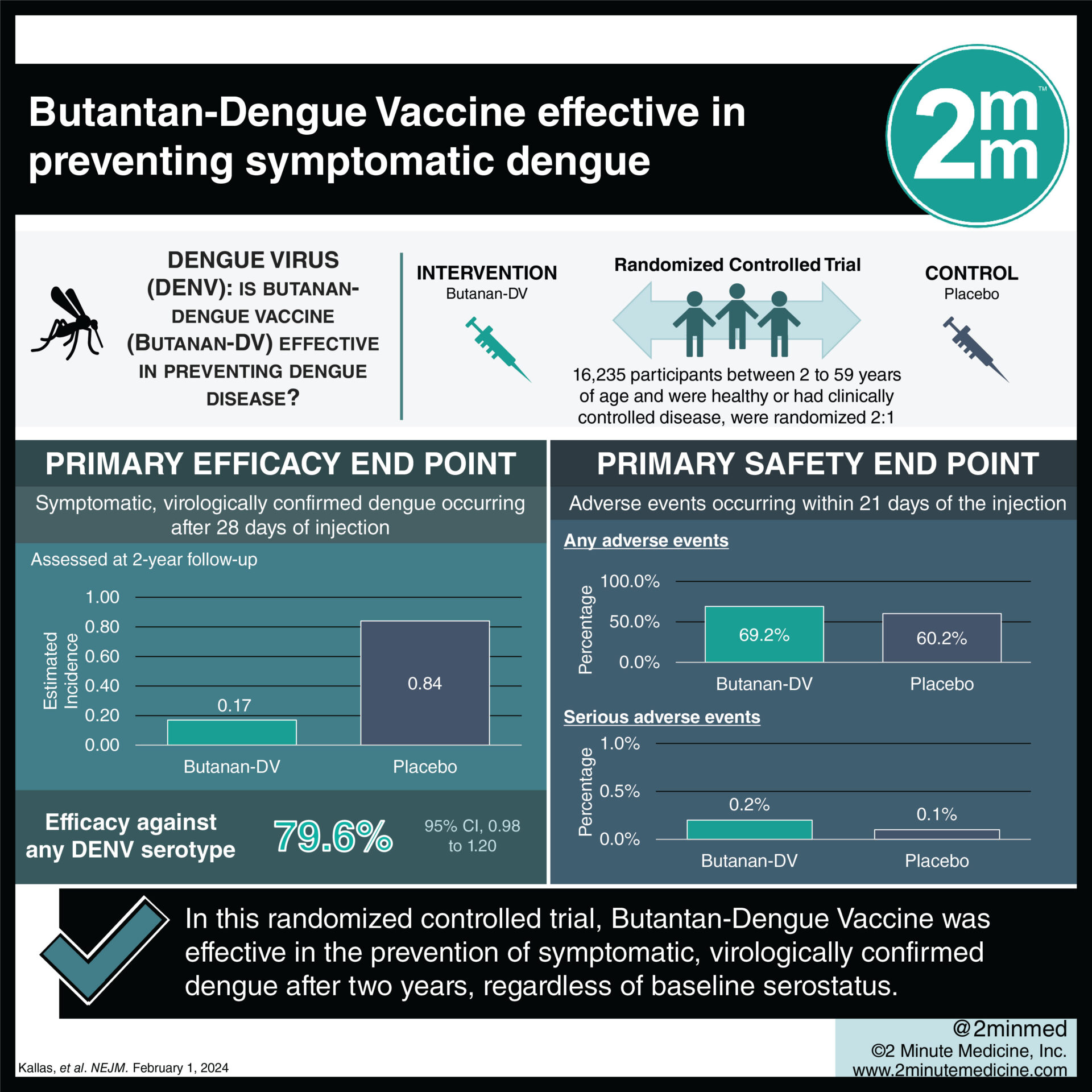
#VisualAbstract: Butantan-Dengue Vaccine effective in preventing symptomatic dengue
1. In this randomized controlled trial, the Butantan-Dengue Vaccine (Butantan-DV) was effective in the prevention of symptomatic, virologically confirmed dengue after two years, regardless of baseline serostatus.
2. Butantan-DV was effective against DENV-1 and DENV-2 serotypes across all age groups.
Evidence Rating Level: 2 (Good)
Study Rundown: Dengue is a mosquito-borne viral illness that can result in high fever and is commonly encountered in Southeast Asia and South and Central America. There are currently two licensed live, attenuated vaccines against dengue, including CYD-TDV and TAK-003, but both require multiple doses and serve a limited age group. Butantan-DV is a single-dose, live, attenuated, tetravalent dengue vaccine candidate that elicited immune responses across all four DENV serotypes and had an acceptable side effect profile regardless of baseline serostatus in a recent phase 2 trial in Brazil. This phase 3 trial investigated the efficacy and safety of Butantan-DV in preventing dengue through two years of follow-up. Overall, the vaccine was effective in the prevention of symptomatic, virologically confirmed dengue after two years, regardless of baseline serostatus. Moreover, it was effective against both DENV-1 and DENV-2 serotypes and across all trial age groups (2 to 6 years, 7 to 17 years, and 18 to 59 years). The incidence of serious adverse events was similar in both the vaccine and placebo groups, although mild-to-moderate adverse events were more common with Butantan-DV. Insights into the vaccine’s efficacy against DENV-3 and DENV-4 are limited given that these serotypes have not been observed during the follow-up period, and long-term data and analysis through five years of follow-up, as recommended by the World Health Organization, is needed.
Click to read the study in NEJM
Relevant Reading: Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy
In-Depth [randomized controlled trial]: DEN-03-IB is an ongoing phase three, double-blind, randomized controlled trial in Brazil, with five years of planned follow-up, which aimed to investigate the efficacy and safety of Butantan-DV against symptomatic dengue through two years of follow-up. Participants were eligible for the trial if they were between 2 to 59 years of age and were healthy or had clinically controlled disease. Participants were stratified into three age groups (2 to 6 years, 7 to 17 years, and 18 to 59 years) and randomized in a 2:1 ratio to receive a single dose of Butantan-DV or placebo. A blood sample prior to injection was obtained to determine each participant’s baseline serostatus. The primary efficacy endpoint was symptomatic, virologically confirmed dengue occurring after 28 days of injection. Secondary endpoints included confirmed dengue according to viral serotype and baseline serostatus. A total of 16,235 participants underwent randomization (10,259 in the vaccine group and 5,976 in the placebo group), and nearly half of these individuals had negative serostatus. Overall, vaccine efficacy against any DENV serotype was 79.6% (95% Confidence Interval [CI], 70.0 to 86.3). Vaccine efficacy against DENV-1 and DENV-2 were 89.5% (95% CI, 78.7 to 95.0) and 69.6% (95% CI, 50.8 to 81.5), respectively. Vaccine efficacy against any serotype among those without previous dengue exposure was 73.6% (95% CI, 57.6 to 83.7), whereas that among individuals with previous exposure was 89.2% (95% CI, 77.6 to 95.6). The incidence of serious adverse events occurring within 21 days of the injection was 0.2% and 0.1% in the vaccine and placebo groups, respectively. In summary, this phase 3 trial demonstrated that a single dose of Butantan-DV was safe and effective in preventing symptomatic DENV-1 and DENV-2 through 2 years of follow-up, regardless of baseline dengue serostatus.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.