#VisualAbstract: Daprodustat noninferior to darbepoetin alfa in the treatment of anemia in patients with chronic kidney disease on incident dialysis
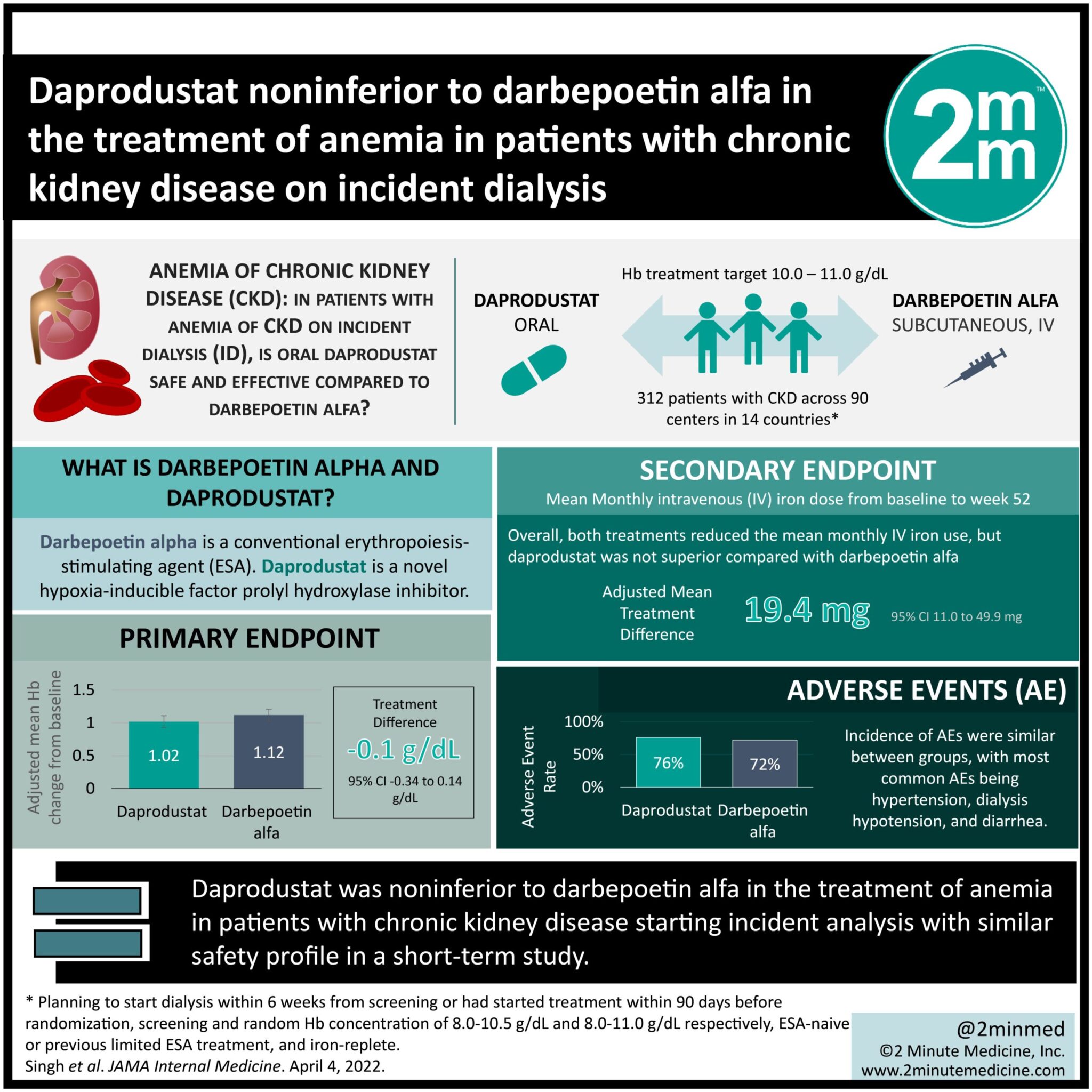
1. This trial demonstrated noninferiority of daprodustat to darbepoetin alfa in the treatment of anemia in patients with chronic kidney disease starting incident analysis.
2. The safety profile was similar between daprodustat and darbepoetin alfa in this short-term study.
Evidence Rating Level: 1 (Excellent)
Study Rundown: In the treatment of patients with anemia of chronic kidney disease (CKD) on incident dialysis (ID), there is limited research examining the comparative efficacy and safety of conventional erythropoiesis-stimulating agents (ESAs), such as darbepoetin alfa and the novel hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs), such as daprodustat. This randomized clinical trial (ASCEND-ID) assessed the efficacy and safety of oral daprodustat compared to darbepoetin alfa for 52 weeks in patients on ID. The primary endpoint was noninferiority of daprodustat compared to darbepoetin alfa (noninferiority margin, −0.75 g/dL) assessed via the mean change in hemoglobin (Hb) concentration from baseline to evaluation period (weeks 28-52). The secondary endpoint was the mean monthly intravenous (IV) iron dose from baseline to week 52. Among 312 patients with CKD on ID, daprodustat was noninferior to darbepoetin alfa in the treatment of anemia of CKD. Furthermore, the safety profile and monthly IV iron usage was similar between treatment groups in this short-term study. Long-term validation trials are required in the future to confirm daprodustat as a potential oral alternative to conventional ESAs for patients with CKD who are starting dialysis. A limitation of this study was the short study period and small sample size, limiting the evaluation of long-term effects and safety associated with daprodustat.
Click to read the study in JAMA Internal Medicine
Relevant Reading: Prolyl-hydroxylase inhibitors for the treatment of anemia in chronic kidney disease
In-Depth [randomized clinical trial]: This multicenter trial included 312 patients (median [IQR] age, 55 [45-65] years; 194 [62%] male) with CKD randomized 1:1 to daprodustat (N =157) or darbepoetin alfa (N = 155) from May 2017 to September 2020 across 90 centers in 14 countries. Eligibility criteria included patients planning to start dialysis within 6 weeks from screening or had started treatment within 90 days before randomization, screening and random Hb concentration of 8.0-10.5 g/dL and 8.0-11.0 g/dL respectively, ESA-naive or previous limited ESA treatment, and iron-replete. The mean (SD) Hb concentration was 10.5 (1.0) g/dL and 10.6 (0.9) g/dL for daprodustat and darbepoetin alfa respectively (adjusted mean treatment difference, −0.10 g/dL [95% CI, −0.34 to 0.14 g/dL]), indicating noninferiority. Overall, both treatments reduced the mean monthly IV iron use, however, daprodustat was not superior compared with darbepoetin alfa in reducing monthly IV iron use (adjusted mean treatment difference, 19.4mg [95%CI, –11.0 to 49.9mg]). Adverse event rates were 76% vs 72% for daprodustat and darbepoetin alfa respectively.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.