#VisualAbstract: Etrasimod improves clinical remission rates in patients with moderate-severe ulcerative colitis
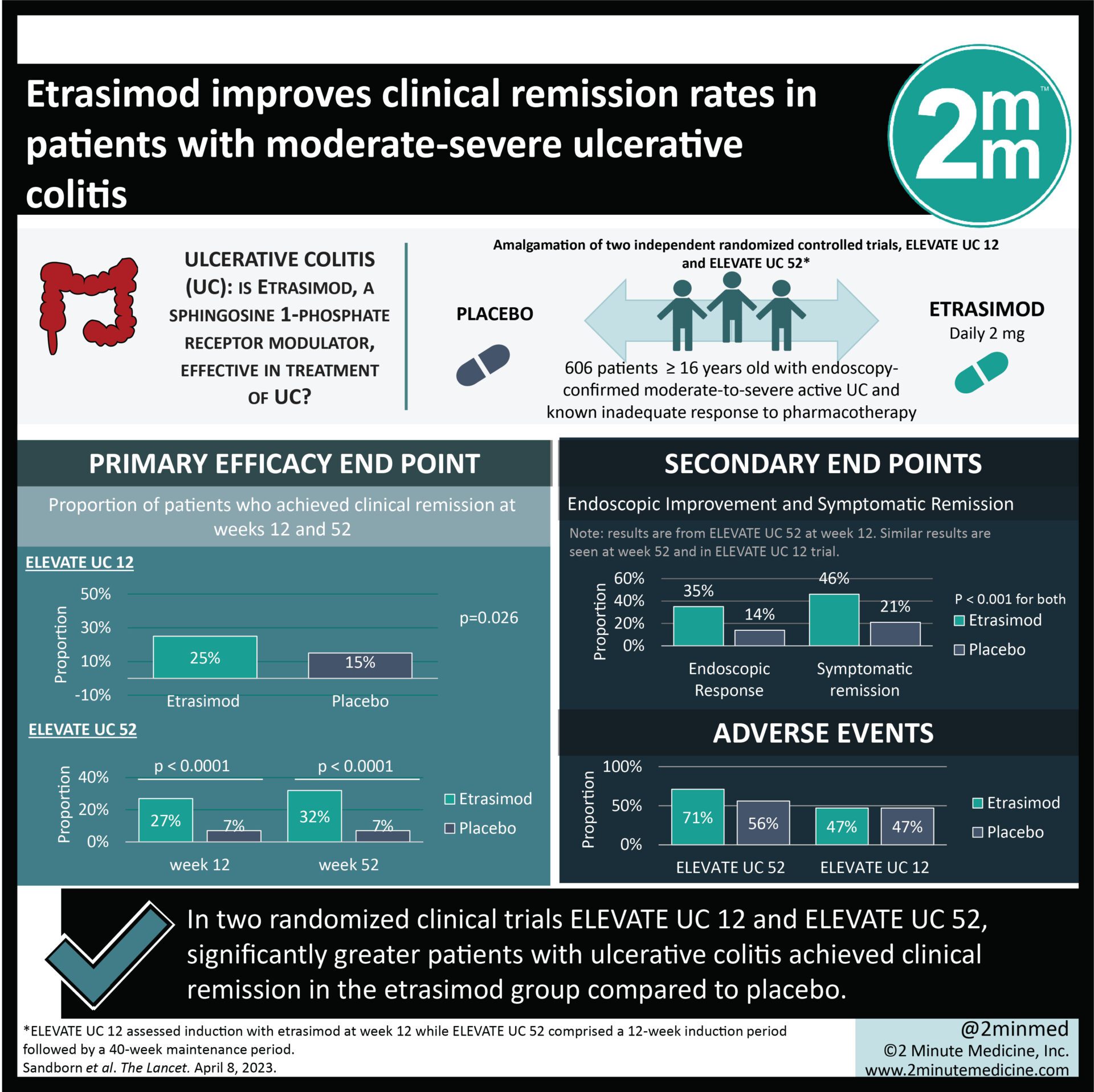 1. In both ELEVATE UC 12 and ELEVATE UC 52, significantly greater patients in the etrasimod group achieved clinical remission compared to placebo.
1. In both ELEVATE UC 12 and ELEVATE UC 52, significantly greater patients in the etrasimod group achieved clinical remission compared to placebo.
2. Majority of adverse events were mild-to-moderate in nature with no reported fatalities.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Ulcerative colitis is a chronic, autoimmune disease of the gastrointestinal tract that affects many patients worldwide. Etrasimod, a sphingosine 1-phosphate receptor modulator, may play a role in treating ulcerative colitis, although little is known to date. This study is an amalgamation of two independent, randomized controlled trials, ELEVATE UC 12 and ELEVATE UC 52, that aimed to assess the safety and efficacy of etrasimod in patients with moderate-to-severe ulcerative colitis. ELEVATE UC 12 assessed induction with etrasimod at week 12 while ELEVATE UC 52 comprised a 12-week induction period followed by a 40-week maintenance period. The primary efficacy endpoint was the proportion of patients who achieved clinical remission at weeks 12 and 52. According to study results, more patients in the etrasimod group achieved clinical remission compared to placebo after 12 weeks of induction and this was sustained up to 52 weeks. Additionally, the etrasimod group reported greater improvements in endoscopic appearance, symptom control, and corticosteroid-free remission. This study was strengthened by data from two independent trials, thus adding to the validity and generalizability of findings.
Click to read the study in The Lancet
Relevant Reading: Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis
In-depth [randomized-controlled trial]: Between Jun 13, 2019, and Jan 28, 2021, 821 patients and 606 patients were assessed for eligibility in ELEVATE UC 52 and ELEVATE UC 12, respectively. Included were patients ≥ 16 years old with endoscopy-confirmed moderate-to-severe active ulcerative colitis and a known history of inadequate response to pharmacotherapy. Patients were randomized to receive daily oral estrasimod 2 mg or placebo. The primary efficacy endpoint of clinical remission was achieved among significantly greater patients in the etrasimod group compared to placebo (25% vs. 15%, p=0.026) in ELEVATE UC 12 after induction for 12 weeks. Similarly, estrasimod improved clinical remission at weeks 12 (27% in etrasimod vs. 7% in placebo, p<0.0001) and week 52 (32% in etrasimod vs. 7% in placebo, p<0.0001) among patients in the ELEVATE UC 52 group. Adverse events were comparable between both groups and there were no treatment-related fatalities. Findings from this study suggest that etrasimod is effective for induction and maintenance in patients with ulcerative colitis.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.