#VisualAbstract: Immunotherapy plus stereotactic radiotherapy improves outcomes in early-stage lung cancer
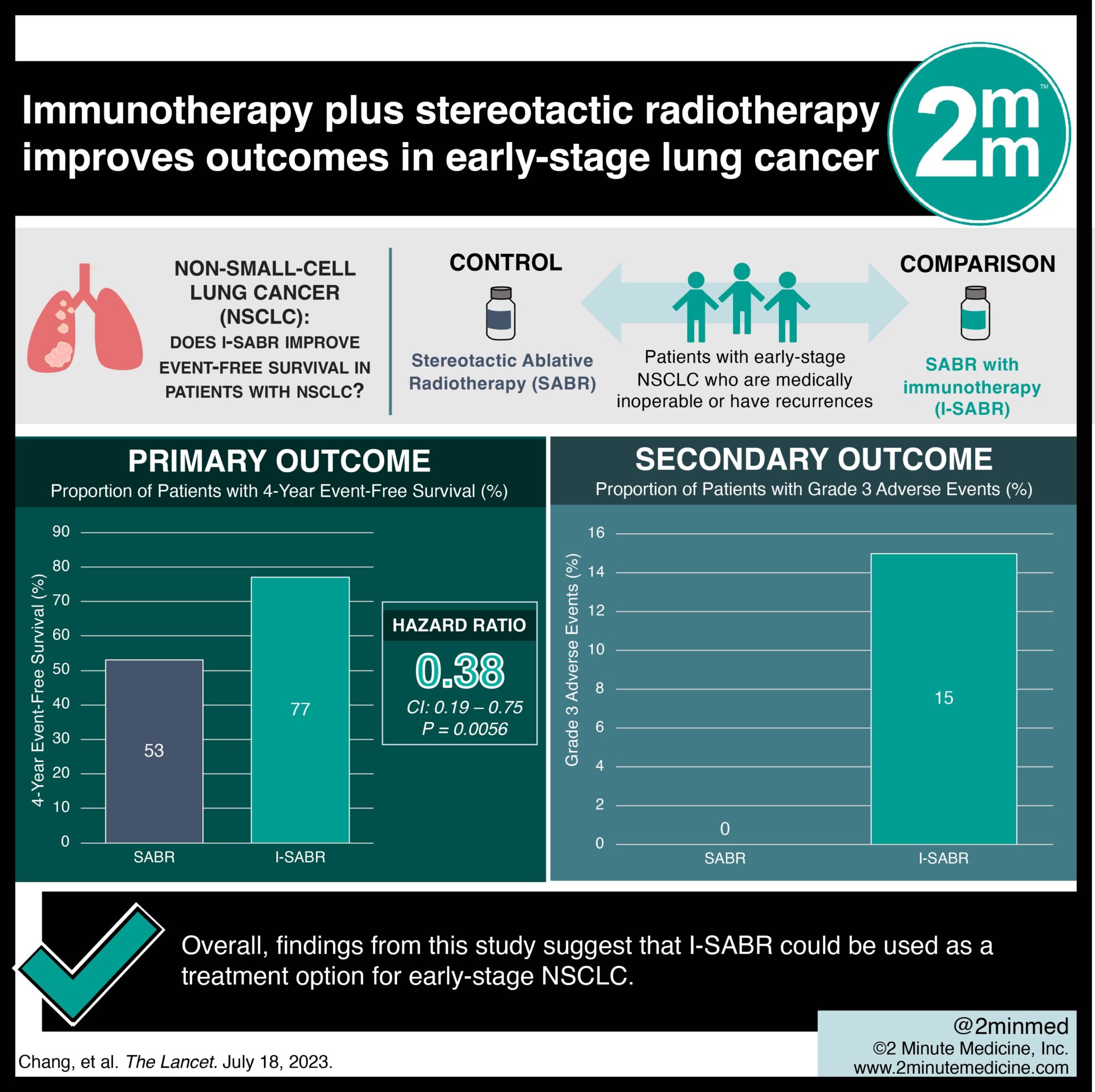 1. 4-year event-free survival was significantly greater in I-SABR (77%) versus SABR alone (53%).
1. 4-year event-free survival was significantly greater in I-SABR (77%) versus SABR alone (53%).
2. More patients in the I-SABR group experienced grade 3 immunological adverse events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Standard treatment for early-stage inoperable non-small-cell lung cancer (NSCLC) includes stereotactic ablative radiotherapy (SABR). However, relapses remain common. Although immunotherapy may has shown efficacy in stage III NSCLC, its efficacy in earlier stages remains unknown. This randomized controlled trial aimed to compare the outcomes of SABR alone with SABR combined with immunotherapy (I-SABR) in early-stage NSCLC patients. The primary outcome was four-year event-free survival, while key secondary outcome was the assessment of adverse events associated with treatment. According to study results, I-SABR significantly improved event-free survival at four years in people with early-stage NSCLC, compared to SABR alone. Although this study was well done, it was limited by its phase 2 nature, and further confirmation with larger phase 3 trials is warranted.
Click to read the study in The Lancet
Relevant Reading: Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer
In-depth [randomized-controlled trial]: Between Jun 30, 2017, and Mar 22, 2022, 183 patients were assessed for eligibility across three hospitals in Texas, USA. Included were patients ≥ 18 years with early-stage NSCLC (IA-IB, IIA-IIB) who were medically inoperable or had isolated parenchymal recurrences. Altogether, 156 patients (78 each in SABR and I-SABR) were included in the final analysis. The primary outcome of 4-year event-free survival was significantly greater in the I-SABR group (77%) compared to SABR alone (53%; hazard ratio 0.38, 95% CI 0.19-0.75; p=0.0056). Moreover, the secondary outcome showed that I-SABR offered improved event-free survival in early-stage NSCLC with tolerable toxicity. More patients in the I-SABR group experienced grade 3 immunological adverse events related to nivolumab (15% in I-SABR vs. 0% in SABR). Overall, findings from this study suggest that I-SABR could be used as a treatment option for early-stage NSCLC.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.