#VisualAbstract: Interleukin-23 receptor antagonist improves psoriasis symptoms
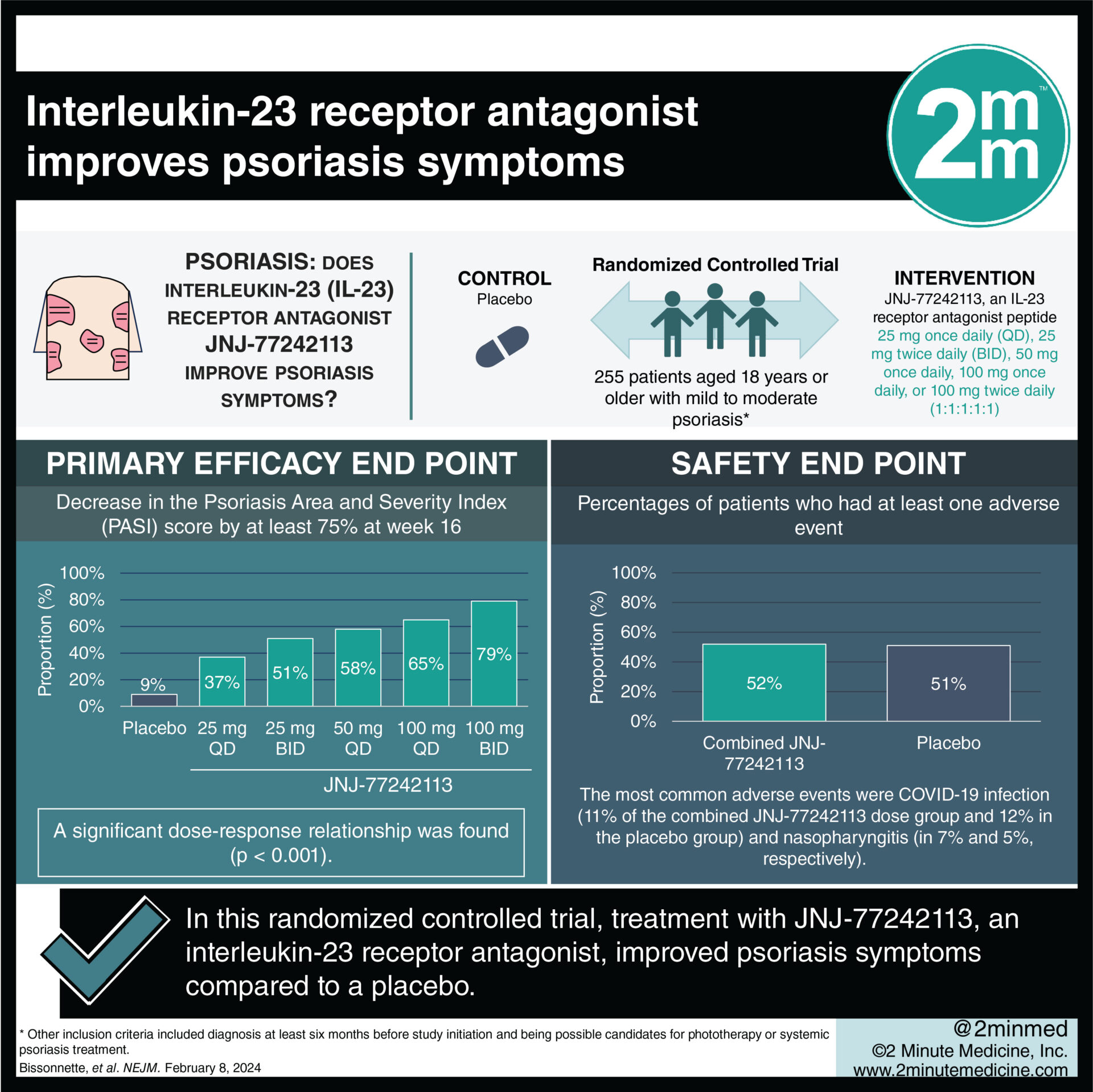
1. In this randomized controlled trial, treatment with JNJ-77242113 improved psoriasis symptoms as compared to a placebo.
2. JNJ-77242113 showed a dose-response relationship, with higher doses eliciting stronger treatment effects.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Psoriasis is an immune-mediated inflammatory disease primarily affecting the skin and joints. The immune process is mediated by Interleukin-23, which plays a key role in T-cell activation. In the past, biologics have been used as treatments for psoriasis; however, they have limitations. Their main limitation is that there is a requirement for intravenous or subcutaneous administration. This study identified the need for a new oral effective therapy for moderate-to-severe psoriasis and proposed using JNJ-77242113, an interleukin-23-receptor antagonist. Randomization placed participants according to a 1:1:1:1:1:1 ratio to receive JNJ-77242113 at an interval of 25 mg once daily, 25 mg twice daily, 50 mg once daily, 100 mg once daily, or 100 mg twice daily or placebo for 16 weeks. The main endpoint was a decrease in the Psoriasis Area and Severity Index (PASI) score by at least 75% at week 16. The effects of JNJ-77242113 were similar to those of several injectable biologic agents currently being used for psoriasis. The trial was limited by the short time and small amount of patients involved. Ultimately, in patients with moderate-to-severe psoriasis, JNJ-77242113 had a dose-response relationship with greater efficacy than the placebo.
Click here to read the study in the NEJM
In-Depth [randomized controlled trial]: This randomized control trial aimed to assess the impact of JNJ-77242113 on psoriasis symptoms in 255 patients with a diagnosis of moderate-to-severe psoriasis. Participants were 18 years or older, had been diagnosed at least six months before study initiation, and were possible candidates for phototherapy or systemic psoriasis treatment. Patients who have been previously treated with a biologic agent targeting interleukin-23 were excluded. The mean duration of psoriasis in the participants was 18.2±12.79 years with the mean PASI score being 19.05±5.83. There was a greater percentage of patients with severe psoriasis, as defined by an IGA score of four, in the JNJ-77242113 25 mg once daily and the 100 mg twice daily groups when compared to all other groups. At 16 weeks, a significant dose-response signal was found for the PASI 75 response (p<0.001). Of patients taking the highest dose, 100 mg twice daily, 79% of them had a PASI 75 response whereas only 9% of patients on the placebo had a response. The percentages of patients with a PASI 75 response were 37% for 25 mg once daily, 51% for 25 mg twice daily, 58% for 50 mg once daily, 65% for 100 mg once daily, 79% for 100 mg twice daily, and 9% for the placebo group. Compared to the placebo group, all of the treatment groups showed lower serum levels of hBD-2. Overall, JNJ-77242113 was more effective than the placebo in controlling moderate-to-severe psoriasis.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.