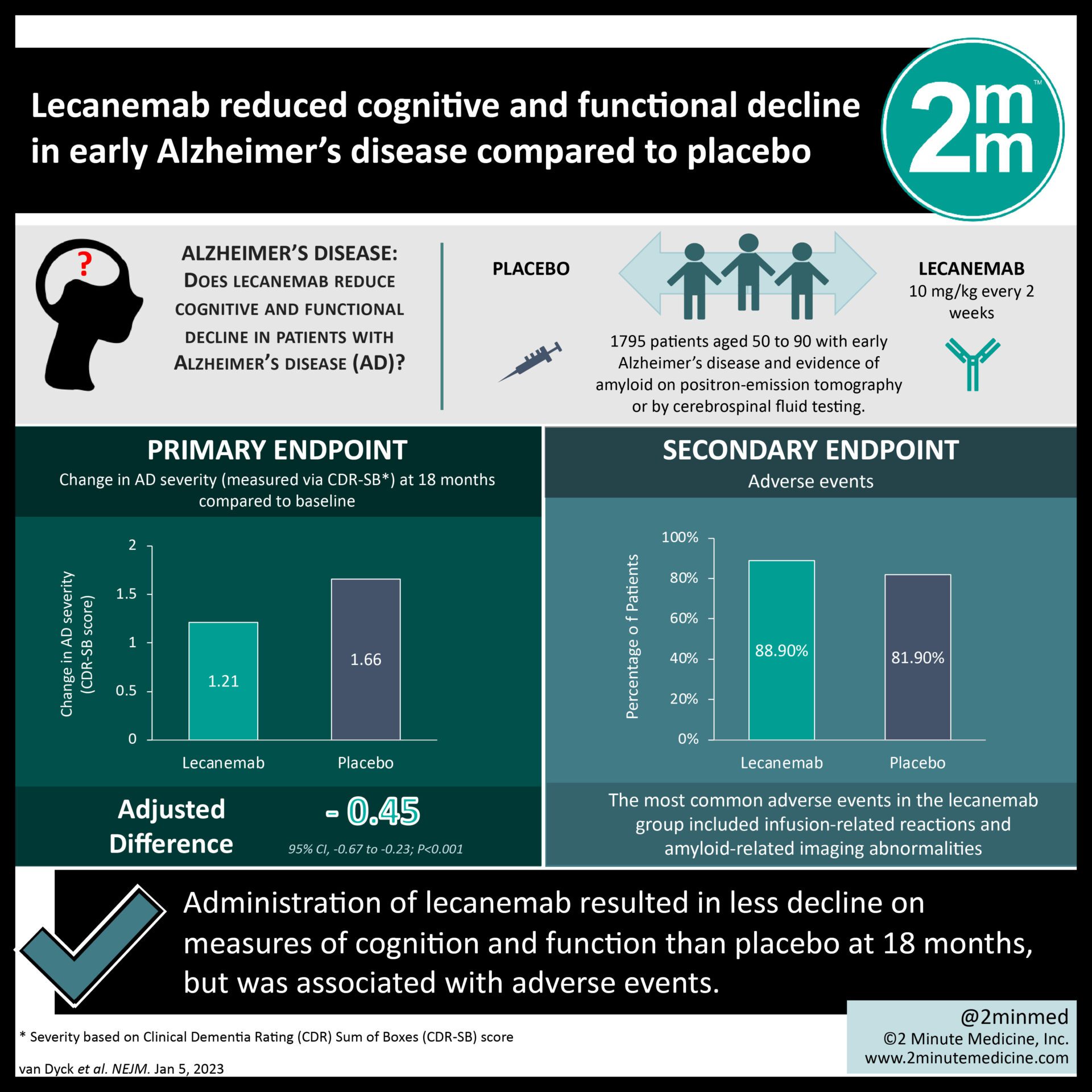
#VisualAbstract: Lecanemab reduced cognitive and functional decline in early Alzheimer’s disease compared to placebo
 1. Lecanemab was associated with reduced amyloid markers in early Alzheimer’s disease as compared to placebo.
1. Lecanemab was associated with reduced amyloid markers in early Alzheimer’s disease as compared to placebo.
2. Lecanemab also resulted in moderately less decline in cognitive and functional measures at 18-month follow-up as compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Rates of Alzheimer’s disease are increasing annually, introducing a considerable burden to patients and the healthcare system. Lecanemab is a humanized monoclonal antibody that binds to soluble amyloid-beta protofibrils. Previously, a phase 2b trial showed no significant difference between lecanemab and placebo at 12 months in patients with Alzheimer’s disease. Though, analyses at 18 months showed less clinical decline on some measures than placebo. However, there is a gap in knowledge as to understanding the safety and efficacy of lecanemab in patients with early Alzheimer’s disease. Overall, this study found that in persons with early Alzheimer’s disease, lecanemab was associated with reduced brain amyloid levels as well as moderately less decline in clinical measures of cognition and function than placebo at 18 months. Notably, lecanemab was associated with an increased number of adverse events. This study was limited by short follow-up time (18 months), having a dropout rate of 17.2%, and some participants and investigators being aware of the trial-group assignments. Nevertheless, these findings are significant, as they demonstrate that lecanemab may provide cognitive and functional benefits in patients with early Alzheimer’s disease.
Click to read the study in NEJM
Relevant Reading: Controversy and Progress in Alzheimer’s Disease — FDA Approval of Aducanumab
In-Depth [randomized controlled trial]: This multicenter, double-blind, placebo-controlled, parallel-group trial was conducted to determine the efficacy of lecanemab in treating patients with early Alzheimer’s disease. Patients who were between 50 to 90 years of age with mild cognitive impairment due to Alzheimer’s were eligible for the study. All participants also had objective impairment in episodic memory as indicated by at least one standard deviation below the age-adjusted mean in the Wechsler Memory Scale IV–Logical Memory II. Patients who did not fit these inclusion criteria were excluded from the study. Patients were followed for a total of 18 months. The primary outcome measured was the change in the score on the Clinical Dementia Rating (CDR)–Sum of Boxes (CDR-SB)18 from baseline to 18 months. Outcomes in the primary analysis were assessed via efficacy analyses in the modified intention to treat population. Based on the primary analysis, the adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, -0.45; 95% confidence interval [CI], -0.67 to -0.23; p<0.001). In a sub-study involving 698 participants, there were greater reductions in brain amyloid burden with lecanemab than with placebo (difference, -59.1 centiloids; 95% CI, -62.6 to -55.6). However, lecanemab resulted in infusion-related reactions in 26.4% of participants and amyloid-related imaging abnormalities with edema or effusions in 12.6% of participants. Overall, this study demonstrates that lecanemab was associated with reduced markers of amyloid in early Alzheimer’s disease and resulted in less decline in cognitive and functional measures than placebo at 18 months but was associated with significant adverse effects.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.