#VisualAbstract: Low dose edoxaban prevents stroke in elderly patients with atrial fibrillation
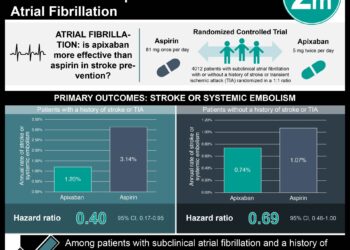
1. Low dose edoxaban was superior to placebo in preventing stroke or systemic embolism for very elderly patients with nonvalvular atrial fibrillation at risk of bleeding.
2. Edoxaban did not significantly increase the incidence of major bleeding compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Atrial fibrillation and age are independent risk factors for stroke, and atrial fibrillation prevalence increases with age. Clinical guidelines for stroke prevention in patients with atrial fibrillation state the use of direct oral anticoagulants. However, physicians are unwilling to prescribe direct oral anticoagulants to elderly patients due to the perceived risk factors for bleeding such as renal failure and previous falls. As such, this study assessed low dose edoxaban for stroke prevention and the risk of bleeding in very elderly patients with nonvalvular atrial fibrillation. The results of the study demonstrated that a once-daily, 15 milligram dose of edoxaban was superior to the placebo with respect to preventing strokes without significantly increasing the incidence of major bleeding. This randomized trial was limited by study adherence. A large number of participants discontinued the trial due to adverse events unrelated to bleeding or were unable to continue their participation, with equal number withdrawing in both groups. Nonetheless, this study’s results are significant and its findings highlight the effects of the medication on participants with high-risk backgrounds.
Click to read the study in NEJM
Relevant Reading: Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease
In-Depth [randomized controlled trial]: This randomized control trial enrolled 984 participants in a multicenter study at 164 institutions in Japan. Participants included in the study were at least 80 years of age and had a history of nonvalvular atrial fibrillation. Participants with a CHAD2 score of 1 were excluded from this study. The participants were randomized in a 1:1 ratio to receive a once-daily, 15 milligram dose of edoxaban or placebo, respectively. The primary efficacy end point was the composite of stroke or systemic embolism. The mean (±SD) age of the participants was 86.6±4.2 years. Stroke or system embolism occurred in 15 participants (2.3% per patient-year) in the edoxaban group compared to 44 participants (6.7% per patient-year) in the control group (hazard ratio, 0.34; 95% confidence interval [CI], 0.19 to 0.61; P<0.001). Furthermore, there were 66 reported deaths from any cause in the edoxaban group (9.9% per patient-year) compared to 69 deaths in the control group (10.2% per patient-year; hazard ratio, 0.997; 95% CI, 0.69 to 1.36). The annualized rate of major cardiovascular events was 7.7% in the edoxaban group compared to 11.0% in the control group (hazard ratio, 0.70; 95% CI, 0.49 to 1.01). In regard to safety, there were 20 major bleeding events (3.3% per patient-year) in the edoxaban group compared to 11 events (1.8% per patient-year) in the control group (hazard ratio, 1.87; 95% CI, 0.90 to 3.89; P=0.09). More events of gastrointestinal bleeding occurred in the edoxaban group (14 events; 2.3% per patient-year) compared to the control group (5 events; 0.8% per patient-year; hazard ratio, 2.85; 95% CI, 1.03 to 7.88). Finally, there were no fatal bleeding events in the edoxaban group compared to the two events in the control group. Taken together, a once-daily, 15 milligram dose of edoxaban was superior to the placebo for stroke and systemic embolism prevention without significantly increasing the risk of major bleeding events in very elderly patients with nonvalvular atrial fibrillation.
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.