#VisualAbstract: Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes
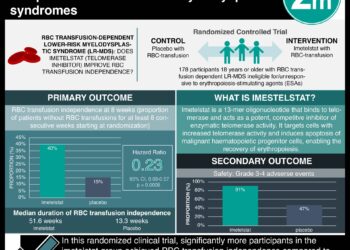
1. Luspatercept may significantly reduce transfusion burden in patients with lower-risk myelodysplastic syndromes with ring sideroblasts for whom erythropoiesis-stimulating agents have not been effective.
Evidence Rating: 1 (Excellent)
Myelodysplastic syndromes are acquired bone marrow disorders characterized by ineffective hematopoiesis, progressive cytopenias and an increased risk of acute myeloid leukemia, predominantly seen in the elderly population. Lower risk myeodysplastic syndromes most commonly manifest as symptomatic anemia, and are associated with cardiovascular complications, falls, and shorter survival. A large proportion of these patients eventually become dependent on red-cell transfusions, which are associated with reduced quality of life and overall survival. Currently, erythropoiesis-stimulating agents are first-line treatments for lower-risk myelodysplastic syndromes, and are used to increase the duration of transfusion independence. Alternative treatment options are limited to use in certain patient populations such as patients with myelodysplastic syndromes with ring sideroblasts, who tend to remain responsive to stimulating agents for shorter durations of time. In this double-blind, placebo-controlled, phase 3 trial, 229 patients with very-low risk, low-risk or intermediate-risk myelodysplastic syndrome with sideroblasts who had been receiving regular red-call transfusions were randomized to receive luspatercept (1.0 to 1.75 mg/kg) or placebo administered subcutaneously every 3 weeks to study the efficacy of luspatercept as a novel treatment option, with transfusion independence being the primary end point. Researchers found that patients assigned to receive luspatercept had a significantly higher rate of transfusion independence for 8 weeks or longer (38%) compared to the placebo group (13%) during weeks 1 to 24 (p<0.001). Findings persisted with the experimental group having a higher likelihood of being transfusion independent at 12 weeks or longer through weeks 1 to 24 and 24 to 48 (p<0.001 for both comparisons). This study therefore shows that in patients with lower-risk myelodysplastic syndromes with ring sideroblasts, luspatercept may represent a promising treatment option where erythropoiesis-stimulating agent therapies are ineffective.
Click to read the study in NEJM
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.