#VisualAbstract: Malaria Chemoprevention Improves Postdischarge Management of Severe Anemia
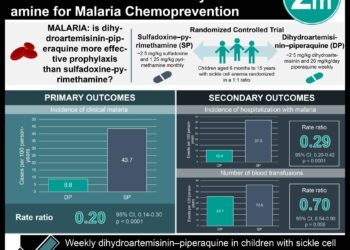
1. Dihydroartemisinin-piperaquine was shown to prevent deaths of children with severe anemia in areas with intense malaria transmission compared to placebo.
2. Monthly chemoprevention with dihydroartemisinin-piperaquine was shown to reduce readmissions for any reason in children with severe anemia compared to placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Severe anemia substantially contributes to childhood mortality and hospital admissions in malaria endemic areas of Africa. Most work of severe anemia in Africa focuses on improving in-hospital care; however, a considerable burden is maintained in the first few months after discharge. Specifically, more deaths occur during the period of discharge compared to the in-hospital care period. As such, this study assessed the efficacy of a three-month post-discharge long-acting antimalarial drug combination of dihydroartemisinin-piperaquine in preventing readmission or death in children with severe anemia. The study determined that dihydroartemisinin-piperaquine use led to a prevention in death and readmission for any reason in children compared to placebo. The randomized controlled trial was limited by the lower-than-expected mortality in the placebo group. Study participation provided the children with a standard of care not readily available; thereby, improving survivorship, but not reflecting the actual mortality burden of the infection. Nonetheless, this study’s results are significant, and its findings highlight a preventative method in increasing children survivorship after malarial infection.
Click to read the study in NEJM
Relevant Reading: Immediate transfusion in African children with uncomplicated severe anemia
In-Depth [randomized controlled trial]: This randomized controlled trial enrolled 1,049 participants at nine hospitals in Kenya and Uganda. Participants included in the study were children younger than the age of five years and admitted with severe anemia. Severe anemia was identified as hemoglobin level of <5 grams (g) per deciliter, hematocrit of <15%, or blood transfusion indication not caused by sickle cell disease, cancer, trauma, or elective surgery. Participants with sickle cell disease or a history of cardiac disorders were excluded from this study. The participants were randomized in a 1:1 ratio to receive post-discharge malaria chemoprevention or placebo treatment, respectively. The primary outcome was hospital readmissions for any reason of death. The participants were followed for six months after discharge. At the end of the follow-up period, 138 of 524 participants (26.3%) had a readmission or died in the chemoprevention group compared to 177 of 525 participants (33.7%) in the placebo group. Overall, the risk of readmission or death was 35% lower in the chemoprevention group compared to the placebo group (hazard ratio, 0.65; 95% confidence interval [CI], 0.54 to 0.78; P<0.0001). The readmission or death incidence was higher among participants with severe malarial anemia (41%) compared to children with nonmalarial anemia (9%); however, the difference was not significant. Finally, there were 284 serious adverse events in the chemoprevention group compared to the 534 events in the placebo group (P<0.001). Furthermore, 12 participants in the chemoprevention group (2.3%) and 30 participants in the placebo group (5.7%) experienced the severe adverse events within four days after medication administration. Taken together, the chemoprevention treatment of dihydroartemisinin-piperaquine significantly reduced deaths and readmissions of children with severe anemia in malaria endemic areas of Africa.
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.