#VisualAbstract: Moderately accelerated cardiac pacing may be beneficial in patients with heart failure with preserved ejection fraction
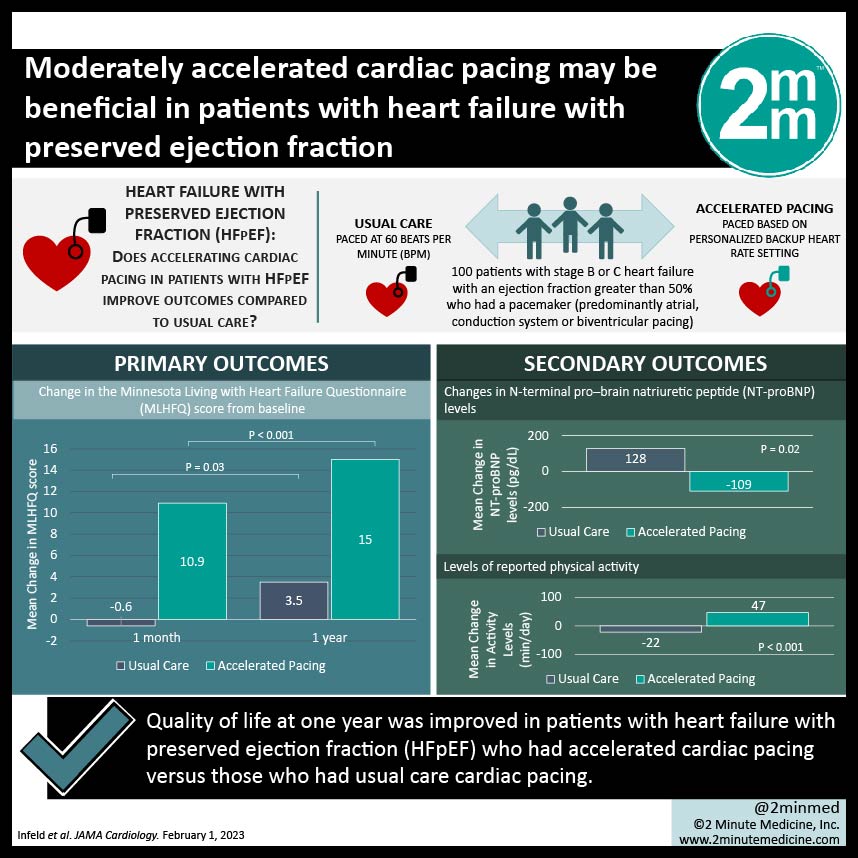
1. Quality of life at one year was improved in patients with heart failure with preserved ejection fraction (HFpEF) who had accelerated cardiac pacing versus those who had usual care cardiac pacing.
2. Accelerated pacing was also associated with lowered B-type natriuretic peptide (BNP) levels and increased activity.
Level of Evidence Rating: 1 (Excellent)
Study Rundown: Heart failure with preserved ejection fraction (HFpEF) is an increasingly common source of morbidity, with very few specific modalities available for treatment. While pharmacologically reducing cardiac pacing with beta-blockers is effective for heart failure with reduced ejection fraction, it is no longer recommended in HFpEF. In the myPACE trial, researchers attempted to determine whether actually accelerating cardiac pacing in patients with HFpEF improves outcomes compared to usual care.
100 patients in total were included in the study, with 48 in the intervention arm and 52 in the usual care arm. The baseline, pre-intervention heart rate was 65 beats per minute (bpm) in each group. After a median follow-up duration of 378 days, patient-reported quality of life as measured by the MLHFQ was significantly higher in the accelerated pacing group compared to the usual care group. MLHFQ scores decreased in the usual care group, reflecting a worse quality of life, while they increased over time in the accelerated pacing group. The paced intervention was also significantly associated with increased levels of reported physical activity compared to declines in the usual care group.
This randomized controlled trial by Infeld et al demonstrated considerable benefit to personalized, accelerated cardiac pacing in individuals with a pacemaker and HFpEF. This relationship was postulated to be secondary to improved cardiac contractility and relaxation in patients with slightly higher-paced heart rates, leading to improved hemodynamics and an overall better quality of life. Strengths of this study include the randomized, blinded design, which does well to control for confounding. Additionally, the magnitude of the associations found in this trial is remarkable and lends strength to the trial’s conclusions. Limitations include the relatively small sample size and intention-to-treat analysis, which may have overestimated treatment effects to some degree. Certainly, these results are promising and should be further interrogated in a more broad setting.
Click here to read this study in JAMA Cardiology
In-Depth [randomized controlled trial]: A prospective, single-center, parallel-group randomized controlled trial was conducted with the trial protocol published a priori. Patients with stage B or C heart failure with an ejection fraction greater than 50% who had a pacemaker (predominantly atrial, conduction system or biventricular pacing) were eligible. The accelerated cardiac pacing intervention was derived from an algorithm which accounts for average resting heart rate by height and ejection fraction. The usual care group was paced at 60 beats per minute (bpm). The study’s primary outcome was patient-reported quality of life as determined by the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
The baseline median heart rate in the intervention group was 65 bpm (interquartile range 61-70), and in the control group was also 65 (65-70). At one month, the mean change in MLHFQ score was -0.6 points (standard deviation 9.1) in the control group and was lowered by 3.5 points (10.6) after a year. Conversely, the mean quality of life score was improved by 10.9 (13.7) points at one month and 15.0 (15.5) and one year in the accelerated pacing group. With regards to secondary outcomes, pro-BNP levels were decreased after one month in the paced group and increased in the usual care group (p = 0.02). Additionally, levels of physical activity were also significantly higher in the intervention group at one month (p = 0.02), demonstrating a decline from the baseline in the control group.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.