#VisualAbstract: Nemolizumab for pruritis in atopic dermatitis patients
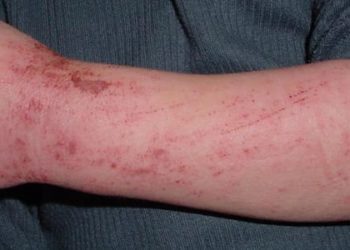
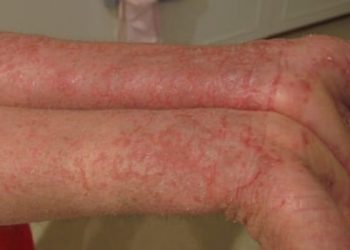
1. In this study, nemolizumab with topical agents and oral antihistamines when compared to topical agents and oral antihistamines alone reduced pruritis in atopic dermatitis patients
2. Among patients in the nemolizumab group, eczema area, quality of life and insomnia improved when compared with placebo.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The use of an interleukin-31 receptor antibody, nemolizumab, has been shown in previous studies to reduce inflammation and epidermal-barrier disruption in atopic dermatitis, which can reduce pruritis. The purpose of this trial was to evaluate the efficacy and safety of the addition of nemolizumab to concomitant topical agents and oral antihistamines for patients with moderate-to-severe pruritis, refractory to topical agents. The results of the study demonstrated that the addition of nemolizumab significantly reduced the pruritis visual-analogue scale (VAS) score after 16 weeks of treatment. Other measurements of quality of life were also significantly improved with nemolizumab such as improvement in the Eczema Area and Severity Index (EASI), Dermatology Life Quality Index (DLQI), and the Insomnia Severity Index (ISI). The main safety profile difference was an increased incidence of injection-related reactions compared to placebo. However, several limitations caution the generalizability of the study. This included the inclusion of only Japanese patients, the short duration of follow-up and the inclusion of only patients above the age of 13 years which excluded an important cohort with atopic dermatitis.
Click to read the study, published today in NEJM
Relevant Reading: IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity
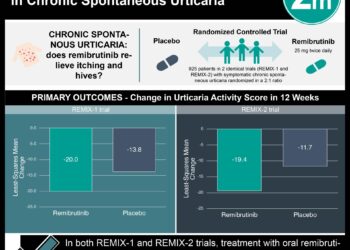
In-Depth [randomized controlled trial]: This 16-week, double-blind, phase 3 trial included 143 Japanese patients with atopic dermatitis, moderate-to-severe pruritis and an inadequate response to topical agents and oral antihistamines. To be included in the study, patients had to be at least 13 years of age and have an inadequate pruritic response to topical glucocorticoids, topical calcineurin inhibitors and oral antihistamines. Patients were randomly assigned in a 2:1 ratio to receive nemolizumab or placebo, with both groups receiving topical agents and oral antihistamines. The primary efficacy endpoint demonstrated that the percent change in pruritis VAS score was -42.8% in the treatment group compared to -21.4% in placebo (95% CI -30.2 to -12.7). Secondary endpoints showed nemolizumab improved the EASI score (-45.9 vs. -33.2 [95% CI -26.6 to -11.9]), increased the percentage of patients with a DLQI score of ≤4 (17% difference [95% CI 2 to 31]), and increased the percentage of patients with an ISI score of ≤7 (33% difference [95% CI 17 to 48]). The most commonly reported adverse event was worsening atopic dermatitis which was present in 24% of the treatment group and 21% in placebo. Cytokine abnormalities such as an increased thymus and activation-regulated chemokine level was observed only in the treatment group but was not associated with worsening atopic dermatitis. Notably, 8% of the nemolizumab group had injection-related reactions compared to 3% in placebo.
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.