#VisualAbstract: Pembrolizumab and chemotherapy combination improves survival in advanced pleural mesothelioma
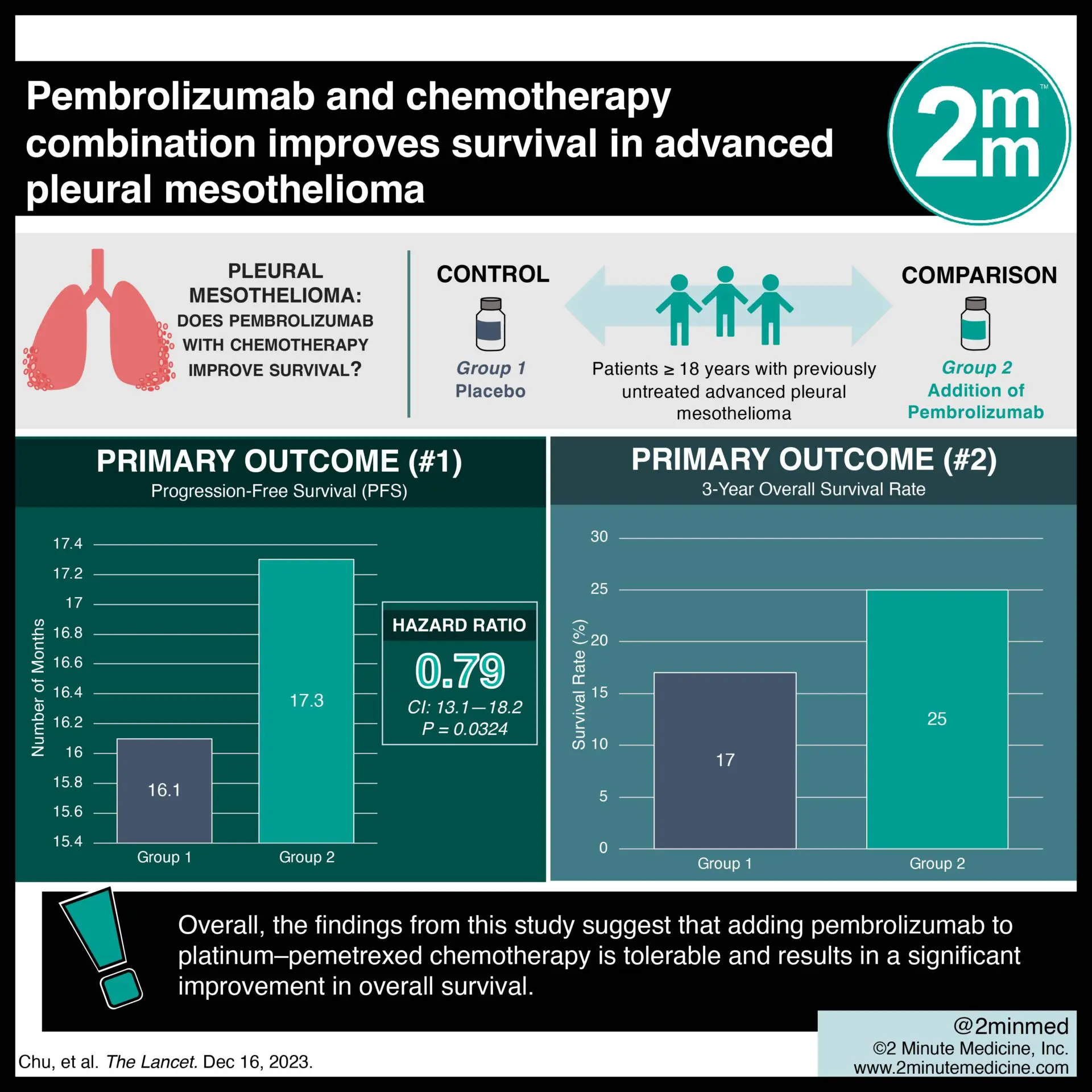
1. Patients in the pembrolizumab plus chemotherapy group reported a significantly greater overall survival compared to those in the chemotherapy alone group (17.3 months vs. 16.1 months).
2. Grade 5 adverse events were comparable in both groups.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Pleural mesothelioma often presents at an advanced stage, and platinum–pemetrexed chemotherapy is a standard treatment. Pembrolizumab, a programmed cell death protein-1 (PD-1) inhibitor, has been used to treat non-small-cell lung cancer; however, little is known about its use in patients with mesothelioma. This randomized controlled trial aimed to evaluate the safety and efficacy of adding pembrolizumab to platinum-based chemotherapy in patients with advanced pleural mesothelioma. The primary outcome was overall survival while key secondary outcome was quality of life. According to study results, pembrolizumab plus chemotherapy showed significantly longer overall survival compared to chemotherapy alone with comparable safety profiles. A major strength of this study was the randomized design with a large patient population from various countries, adding to the generalizability of findings.
Click to read the study in The Lancet
Relevant Reading: Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer
In-depth [randomized-controlled trial]: Between Jan 31, 2017, and Sept 4, 2020, 440 patients were enrolled across 51 hospitals in Canada, Italy, and France. Included were patients ≥ 18 years old with previously untreated advanced pleural mesothelioma and an Eastern Cooperative Oncology Group performance status score ≤ 1. Altogether, 440 patients (222 in chemotherapy plus pembrolizumab and 218 in chemotherapy alone) were included in the final analysis. The primary outcome of overall survival significantly favored pembrolizumab over chemotherapy alone (17.3 months, 95% confidence interval [CI] 14.4-21.3 vs. 16.1 months, 95% CI 13.1-18.2; hazard ratio [HR] 0.79, p=0.0324). Moreover, the secondary outcome of 3-year overall survival rate was 25% with pembrolizumab and 17% with chemotherapy alone. Findings from this study suggest that adding pembrolizumab to platinum–pemetrexed chemotherapy is tolerable and results in a significant improvement in overall survival.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.