#VisualAbstract: Rectal indomethacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients
1. Dose escalation of rectal indomethacin does not provide any benefit in preventing pancreatitis in high-risk patients post-endoscopic retrograde cholangiopancreatography.
Evidence Rating Level: 1 (Excellent)
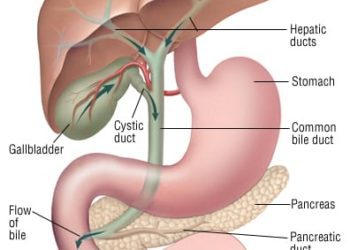
Pancreatitis is the most frequent and concerning complication of endoscopic retrograde cholangiopancreatography (ERCP). Use of rectal non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin and diclofenac peri-procedurally has been shown to produce a reduction in pancreatitis in high-risk patients, and has been adopted into practice. Despite the introduction of prophylactic pharmacotherapy or stent placement, the incidence of pancreatitis in this population remains approximately 10%. The objective of this randomized, double-blind superiority trial was to examine whether a dose escalation strategy with a higher initial dose followed by a second dose of NSAID would be superior relative to standard NSAID dosing for prevention of pancreatitis post-ERCP in high-risk patients. One thousand thirty-seven patients deemed to be at high risk for pancreatitis post-ERCP were enrolled between July 2013 and March 2018 from six American tertiary medical centers. They were randomized post-operatively to either two 50mg indomethacin suppositories and a placebo suppository (standard dose group) or three 50mg indomethacin suppositories with an additional 50mg suppository four hours postoperatively (high-dose group). The primary outcome was pancreatitis post-ERCP. A total of 141 (14%) of 1,037 patients in the trial developed pancreatitis post-operatively. Pancreatitis occurred in 76 (15%) of 515 participants in the standard dose group and 65 (12%) of 522 patients in the high-dose group (RR 1.19, 95% CI 0.87 to 1.61, p = 0.32). Nineteen study drug-related adverse events were reported, with clinically significant bleeding (n=14) being most common, and having comparable incidences between groups (1% vs. 1% in standard dose and high-dose groups). Other adverse events included acute kidney injury (0% vs. 1%), non-ST elevation myocardial infarction (<1% vs. 0%) and transient ischemic attack (<1% vs. 0%). Study findings therefore suggest no significant benefit with dose escalation of rectal indomethacin for pancreatitis prophylaxis in high-risk patients post-ERCP and do not warrant a change in current clinical practice.
Click to read the study in Lancet Gastroenterology & Hepatology
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.