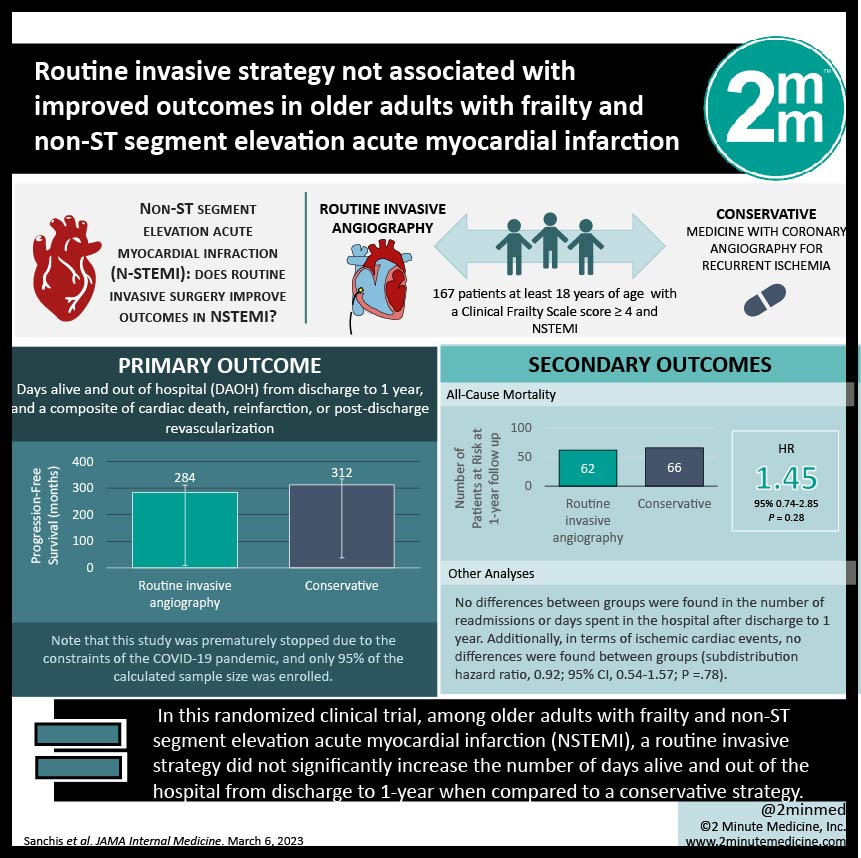
#VisualAbstract: Routine invasive strategy not associated with improved outcomes in older adults with frailty and non-ST segment elevation acute myocardial infarction
1. In this randomized clinical trial, among 167 older adults with frailty and non-ST segment elevation acute myocardial infarction (NSTEMI), a routine invasive strategy did not significantly increase the number of days alive and out of the hospital from discharge to 1-year when compared to a conservative strategy.
2. Days alive and out of the hospital from discharge to 1 year was approximately 1 month greater for patients managed conservatively (312 days) vs patients managed invasively (284 days), and not statistically significant.
Evidence Rating Level:1 (Excellent)
Study Rundown: Frailty is known to negatively impact the prognosis of NSTEMI in older adults. The optimal management of these patients, including invasive or conservative strategies, remains largely unknown. This multicenter randomized clinical trial aimed to compare outcomes of invasive and conservative strategies in frail, older patients with NSTEMI at 1 year. Patients were recruited from 13 Spanish hospitals between July 2017 to January 2021. A total of 167 older adults (≥ 70 years) were randomized to routine invasive (coronary angiography and revascularization; n = 84) or conservative (medical treatment with coronary angiography for recurrent ischemia; n = 83) strategies. The primary outcome was the number of days alive and out of the hospital (DAOH) from discharge to 1-year. DAOH was 28 days greater for patients managed conservatively (312 days) than those managed invasively (284 days). There was a 28-day shorter survival in the invasive vs conservatively managed group. No sex-specific differences in outcomes were found. A limitation of this study was that due to the constraints of the COVID-19 pandemic, only 95% of the estimated sample size was recruited. A major strength of this study was its novel approach to investigating the relationship between invasive vs conservative therapy in frail older adults.
Click to read the study in JAMA Internal Medicine
Click to read an accompanying invited commentary in JAMA Internal Medicine
In-Depth [randomized controlled trial]: This study investigated invasive vs conservative strategies for the management of frail older adults with NSTEMI at 1 year. This multicenter trial was conducted at 13 Spanish hospitals between July 7, 2017, and January 9, 2021. A total of 167 older adults (mean [SD] age, 86 [5] years, and mean [SD] Clinical Frailty Scale score, 5 [1]) with a Clinical Frailty Scale score ≥ 4, and NSTEMI were randomized to receive either routine invasive (coronary angiography and revascularization if feasible; n = 84) or conservative (medical treatment with coronary angiography for recurrent ischemia; n = 83) strategies. The primary endpoints were DAOH from discharge to 1 year, and a composite of cardiac death, reinfarction, or post-discharge revascularization. This study was prematurely stopped due to the constraints of the COVID-19 pandemic, and as a result, only 95% of the calculated sample size was enrolled. DAOH was found to be approximately 1 month (28 days; 95% CI, -7 to 62) greater for patients managed conservatively (312 days; 95% CI, 289 to 335) vs patients managed invasively (284 days; 95% CI, 255 to 311; P = .12). There was a 28-day shorter survival in the invasive vs conservatively managed group (95% CI, -63 to 7 days; restricted mean survival time analysis). 56% of readmissions were due to noncardiac reasons. No differences between groups were found in the number of readmissions or days spent in the hospital after discharge to 1 year. Additionally, in terms of ischemic cardiac events, no differences were found between groups (subdistribution hazard ratio, 0.92; 95% CI, 0.54-1.57; P =.78).
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.


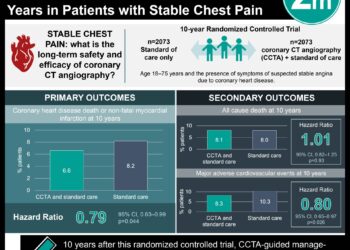
![PFO closure equivalent to medical management in prevention of recurrent stroke [PC and RESPECT trials]](https://www.2minutemedicine.com/wp-content/uploads/2013/03/Echokardiogram_von_Atriumseptumdefekt_Ostium_secundum1-350x250.jpg)