#VisualAbstract: Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients
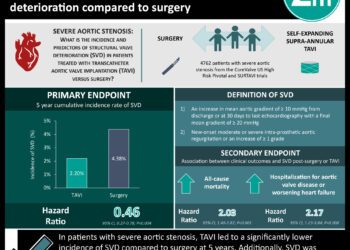
1. Among patients with severe aortic stenosis at low-risk for surgery, transcatheter aortic valve replacement (TAVR) with a self-expanding valve was noninferior to surgical aortic valve replacement (SAVR) with respect to a composite of all-cause mortality or disabling stroke at 24 months.
2. While all-cause mortality was similar between TAVR and SAVR at 24 months, the incidence of disabling stroke, atrial fibrillation, and acute kidney injury was significantly less with TAVR while rates of pacemaker implantation was less in the SAVR group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Though transcatheter aortic valve replacement (TAVR) was originally developed as a treatment option for high-risk patients with severe aortic stenosis, its indications have expanded to intermediate-risk patients on an individualized basis; however, limited evidence exists in the low-risk patient population. The Evolut Low Risk trial was designed to assess the safety and efficacy of TAVR using a self-expandable valve in this low-risk surgical cohort. This reports the results from the prespecified interim analysis of the Evolut trial, which randomized patients with severe aortic stenosis and a three percent or less predicted 30-day surgical mortality risk to either TAVR with a self-expanding valve or surgical aortic valve replacement (SAVR). TAVR was found to be noninferior with respect to the primary endpoint – a composite of death or disabling stroke at 24 months – but did not meet the prespecified criterion for superiority. Among key secondary endpoints, TAVR was found to have a significantly lower incidence of disabling stroke at 24 months, new-onset atrial fibrillation, acute kidney injury (AKI), and severe bleeding. Higher rates of permanent pacemaker implantation and moderate to severe aortic regurgitation were found in the TAVR group. This landmark trial, published alongside results from the PARTNER 3 trial on TAVR with balloon-expandable valves versus surgery, suggests that low-risk surgical patients do as well and perhaps better with TAVR compared to SAVR over two years.
This was the preliminary analysis from a multicenter, randomized trial that used an adaptive Bayesian design. The study provides data for the largest patient cohort currently being treated with SAVR. It is further strengthened by its publication alongside similar results from the PARTNER 3 trial. The main limitation is that only a minority of patients had reached the complete 24-month follow-up. Additional long-term data is needed before making definitive conclusions.
Click to read the study in NEJM
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc