#VisualAbstract: Ubrogepant for the Treatment of Migraine
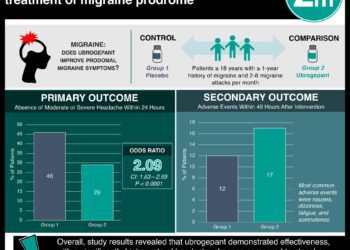
1. In this multicenter study involving adult patients in the United States with a history of migraine, ubrogepant was shown to be more effective than placebo in providing freedom from pain and alleviating the most bothersome symptom within two hours.
2. Doubling the dosage of ubrogepant resulted in limited efficacy benefits but a near-doubling of adverse events, highlighting the necessity of further research into optimal dosage.
Evidence Rating Level: 2 (Good)
Study Rundown: Serotonin receptor agonists (triptans) are currently the standard of care for acute migraine, but small-molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) have shown promise as first or second-line treatment. This study aimed to determine the efficacy, safety, and side-effect profile of oral ubrogepant, finding that the treatment group had a greater percentage of patients with freedom from pain and absence of the most bothersome symptom within 2 hours versus placebo. Patients in the treatment group were also more likely to experience pain relief and sustained pain relief compared to those in the control group. While no adverse events led to discontinuation of the trial regimen, patients who received the 50 mg dose of ubrogepant experienced a lower rate of adverse events versus placebo while patients who received the 100 mg dose experienced a slightly higher rate versus placebo. One strength of this study was its large study population that reflected prevalence in the general population, but the study was limited by its short time frame. Over a fifth of patients who underwent randomization were excluded from the efficacy analysis largely because they did not have a qualifying migraine within a 60-day period, which may have introduced some bias into the final results. In addition, data was collected after only a single attack, meaning that the effects of repeated use were not able to be inferred. Finally, comparative effectiveness was not assessed, limiting the clinical utility of the findings.
Click here to read the study in NEJM
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.