Vitamin D supplementation during winter did not improve psoriasis severity
1. Weekly vitamin D (vitD) supplementation throughout winter did not significantly affect psoriasis severity.
2. Compared to previous studies, 25-hydroxyvitamin D levels increased less than expected in patients receiving vitD supplementation.
Evidence Rating Level: 1 (Excellent)
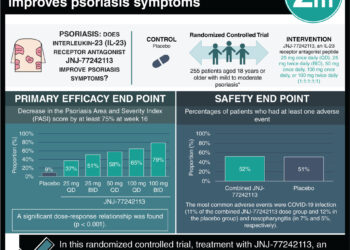
Study Rundown: Vitamin D (vitD) is linked to immune system and keratinocyte proliferation benefits, which are both diminished in psoriasis. Although studies have investigated the effects of vitD supplementation on psoriasis, modification by season has seldom been explored. Thus, this study investigated the benefit of vitD supplementation on psoriasis during two consecutive winter seasons, when ultraviolet exposure was insufficient to confound skin vitD production. The 59 patients randomly assigned to the vitD intervention received a loading dose of 100,000 IU cholecalciferol followed by 20,000 IU per week for 4 months. The control group comprised 61 patients receiving an identical-looking placebo for 4 months. No significant difference in the Psoriasis Area Severity Index (PASI), Physician Global Assessment (PGA) score, self-administered PASI (SAPASI), or Dermatology Like Quality Index (DLQI) was found between the two groups. Compared to previous studies, 25-hydroxyvitamin D levels increased less than expected in the intervention group. Strengths of this study included the randomized controlled design, high compliance, and low attrition. Furthermore, severity scoring was consistent as the same dermatologists completed each assessment. Similar to previous studies, the low baseline severity scores limited the study’s effect assessment. Overall, the results did not support vitD supplementation as a treatment for psoriasis.
Click to read the study in JAMA Dermatology
Relevant Reading: A randomized, double-blind, placebo-controlled trial of the effect of monthly vitamin D supplementation in mild psoriasis
In-Depth [randomized controlled trial]: This randomized controlled study with two parallel groups, approved by the regional ethics committee of North Norway and the Norwegian Medicines Agency, was conducted during two consecutive winters. A total of 122 participants (46 women [37.7%]; mean [SD] age, 53.6 [10.0] years; mean [SD] PASI score, 3.1 [2.0]; mean [SD] serum 25(OH)D, 14.9 [3.9] ng/mL) were recruited from the general Tromsø population and were required to be between 18 to 79 years old with active plaque psoriasis (PASI score > 0) and baseline serum 25(OH)D levels less than 42 ng/mL. Confirmation of inclusion criteria consisted of dermatologist assessment of active plaque psoriasis and blood samples. Exclusion criteria were extensive, including factors such as specific allergies, comorbid diseases, blood pressure values, past treatments, and lifestyle choices. Participant randomization was computer generated based on vitD status, PASI scores, and body mass index, with an allocation ratio of 1:1. In total, 120 participants completed the study. The intervention group included 59 patients (49.2%) receiving a loading dose of 100,000 IU cholecalciferol followed by 20,000 IU per week for 4 months. The control group comprised 61 patients (51.8%) receiving an identical-looking placebo for 4 months. There were four main outcomes: (1) PASI, (2) PGA, (3) SAPASI, and (4) DLQI. Blood samples, medical history, height, weight, hip circumference, waist circumference, and blood pressure were recorded during the first visit. Topical medication was also weighed. Dermatologists assessed psoriasis severity via the PASI and PGA scale, and participants completed the SAPASI and DLQI. Assessments conducted during the first visit were repeated at the fourth-month follow-up, whereas any adverse events were reported during the second and fourth-month follow-ups. Intervention compliance, calculated by the amount of used capsules, was 98.6%. Furthermore, a 25(OH)D level of 30 ng/mL or greater was reached by 24 participants (41.1%). No adverse effects or significant differences between the parallel groups were found among any of the four outcomes: (1) PASI: adjusted difference, 0.11; 95% CI, −0.23 to 0.45; P = .52, (2) PGA: adjusted odds ratio, 0.66; 95% CI, 0.27-1.63; P = .37, (3) SAPASI: adjusted difference, −0.60; 95% CI, −1.76 to 0.55; P = .30, and (4) DLQI: adjusted difference, −0.86; 95% CI, −1.9 to 0.19; P = .11. Overall, this randomized clinical trial did not support vitD supplementation as a treatment for psoriasis.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.