Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma
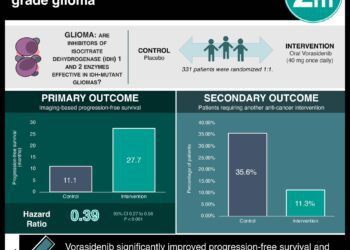
1. Median progression-free survival was shown to be 27.7 months in the vorasidenib group vs 11.1 months in the placebo group, with HR 0.39.
2. Adverse events (grade 3 and above) that were more common with vorasidenib than with placebo were an elevated alanine aminotransferase level, an elevated aspartate aminotransferase level, and an increased γ-glutamyltransferase level.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Gliomas are common malignant brain tumours in adults, with mutations in IDH1 or IDH2 genes present in most grade 2 gliomas. These mutations cause the accumulation of 2-hydroxyglutarate, affecting various processes that enable tumour growth. Treatment often involves chemoradiation but has drawbacks. Vorasidenib, a drug inhibiting mutant IDH1 and IDH2, showed promise in early trials. This study was a phase 3 trial that tested vorasidenib’s efficacy. The primary endpoint was imaged-based progression-free survival (PFS), and secondary outcomes included time to next intervention and safety. Image-based PFS was only assessed in a small group of patients (28% in the treatment group and 54% in the control group), and found that PFS was 27.7 months in the vorasidenib group vs 11.1 months in the placebo group, with HR 0.39. Time to the next intervention in the vorasidenib group as compared with the placebo group showed an HR of 0.26. The likelihood of not receiving the next treatment intervention by 18 months was 85.6% in the vorasidenib group vs 47.4% in the placebo group, and for 24 months was 83.4% vs 27.0%, respectively. With regards to safety, adverse events of grade 3 or higher that were more common with vorasidenib than with placebo were an elevated alanine aminotransferase level (9.6% vs 0%), elevated aspartate aminotransferase level (4.2% vs 0%), and an increased γ-glutamyltransferase level (3.0% vs 1.2%). The strengths of this study included its blinded independent review process, and the limitations included the small sample size, the limited follow-up time, and the low number of patients for whom the primary outcome was assessed. Overall, this study showed some efficacy and low-grade safety profile for vorasidenib for use in IDH-mutant glioma patients post-surgery.
Click to read the study in NEJM
Click to read an accompanying editorial in NEJM
Relevant Reading: Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma
In-Depth [randomized controlled trial]: This international, double-blind, placebo-controlled, phase 3 trial randomized patients with IDH-mutant grade 2 gliomas who had undergone surgery and were considered for a watch-and-wait approach into a treatment arm with vorasidenib (168 patients) and a control arm with placebo (163). The two groups were generally balanced and the median interval between the last glioma surgery and randomization was 2.4 years. Median follow-up was 14.0 months in the vorasidenib group and 14.3 months in the placebo group. Image-based PFS was only assessed in a small group of patients (28% in the treatment group and 54% in the control group) and found that PFS was 27.7 months (95%CI, 17.0-not estimated) in the vorasidenib group vs 11.1 months (95%CI, 11.0-13.7) in the placebo group, with HR 0.39 (95%CI, 0.27-0.56, p<0.001). Time to the next intervention in the vorasidenib group as compared with the placebo group showed an HR of 0.26 (95%CI, 0.15-0.43, p<0.001). The likelihood of not receiving a next treatment intervention by 18 months was 85.6% (95%CI, 77.8-90.8) in the vorasidenib group vs 47.4% (95%CI, 35.8-58.2) in the placebo group, and for 24 months was 83.4% (95%CI, 74.0-89.6) and 27.0% (95%CI, 7.9-50.8), respectively. With regards to safety, adverse events grade 3 or higher were observed in 22.8% of the vorasidenib group and 13.5% in the placebo group. Adverse events of grade 3 or higher that were more common with vorasidenib than with placebo were an elevated alanine aminotransferase level (9.6% vs 0%), elevated aspartate aminotransferase level (4.2% vs 0%), and an increased γ-glutamyltransferase level (3.0% vs 1.2%). This study showed some efficacy and low-grade safety profile for vorasidenib for use in IDH-mutant glioma patients post-surgery.
Image: PD
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.